��Ŀ����
����Ŀ��������һ���ᣬ���ᾧ�壨H2C2O42H2O��������ˮ���۵�ϵͣ����Ȼ��ۻ��������ͷֽ⡣���ᣨH2C2O4�����������Ƶķ�Ӧ��H2C2O4+Ca(OH)2=CaC2O4��(��ɫ)+2H2O��
���������ۣ�
ʵ���ҿ��ü��Ȳ��ᾧ��ֽ�ķ������CO
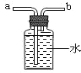
��1���ȼ��Ȳ��ᾧ������CO��CO2��H2O���仯ѧ����ʽ��_____��
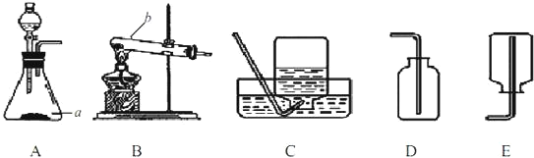
��2���������ͼװ���ռ�CO������Ӧ��_____�˽��루ѡ����a������b������
��ʵ�鷴˼��
��3������Ϊ��ͼ���Թܿ�Ӧ��������б����ʦ��ͬѧ���ۺ�һ����Ϊװ������ȷ�ģ�������_____��
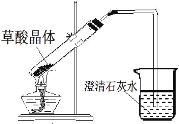
��4��ʵ���й۲쵽����ʯ��ˮ����ǣ�����Ϊһ�����ɲ��ᾧ�����ȷֽ������CO2�����£�����Ϊ�ҵĽ��۲����ܣ�������_____��
���������ӣ�����Ӫ���ḻ�������˶���ͬʳ���ý�ʯ����Ҫ�ɷֲ���ƾ��壩��С��ͬѧ�Բ���ƾ�������ʼ���ɲ�������Ȥ��
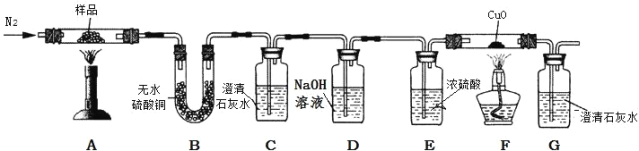
������̽��������ͼװ�ý�����ƾ��壨CaC2O4��xH2O����Ʒ���¼��ȣ�ʹ����ȫ�ֽⲢ������������塣
���������ۣ�
��5��B�й۲쵽_____����˵����Ӧ������ˮ��
��6��C��G�г���ʯ��ˮ������ǣ�˵����Ӧ������_____��_____���塣
��7����ͬѧ��Ϊ�������۲��Ͻ��������ܵó���һ����̼���ɵĽ��ۡ���˵������_____��
�����ȷ����
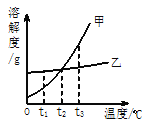
��8�������ȷ����ǶԲ���ƾ��壨CaC2O4��xH2O�������ȷֽ⣬���������ݣ����Ƴɹ����������ֽ��¶ȵĹ�ϵ��ͼ��
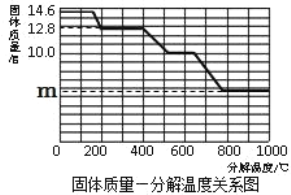
���¶�Ϊ200������ʱ������ȫ��ʧȥ�ᾧˮ�������нᾧˮ������Ϊ_____g��
�ڼ���CaC2O4��xH2O�е�x��CaC2O4����Է���������128����x=_____��
��800������ʱ���������������Ϊ�����ͼ��m��ֵ��_____��
��д������������12.8g��Ϊ10.0gʱ�Ļ�ѧ����ʽ_____��
���𰸡�H2C2O42H2O![]() CO��+CO2��+3H2O�� b �����۵�ϵͣ����ȷֽ�ʱ�����ۻ���ֽ������Թܿ���������б �����Dz�������������ʯ��ˮ�����ɲ���ư�ɫ���� ��ˮ����ͭ���� CO CO2 ������������û�н�������̼��ȫ���� 1.8 1 5.6 CaC2O4
CO��+CO2��+3H2O�� b �����۵�ϵͣ����ȷֽ�ʱ�����ۻ���ֽ������Թܿ���������б �����Dz�������������ʯ��ˮ�����ɲ���ư�ɫ���� ��ˮ����ͭ���� CO CO2 ������������û�н�������̼��ȫ���� 1.8 1 5.6 CaC2O4![]() CaCO3+CO��
CaCO3+CO��
��������
�������ۣ�
��1�����ݡ����Ȳ��ᾧ������CO��CO2��H2O�������仯ѧ����ʽ��H2C2O42H2O![]() CO��+CO2��+3H2O����
CO��+CO2��+3H2O����
��2��CO��������ˮ���ܶȱ�ˮС����ͼ1װ����ˮ���ռ�CO������Ӧ��b�˽��룻
ʵ�鷴˼��
��3������Ϊͼ2���Թܿ�Ӧ��������б��ԭ���Dz����۵�ϵͣ����ȷֽ�ʱ�����ۻ���ֽ⣬�����Թܿ���������б��
��4��ʵ���й۲쵽����ʯ��ˮ����ǣ�����Ϊһ�����ɲ��ᾧ�����ȷֽ������CO2�����£�����Ϊ�ҵĽ��۲����ܣ������Dz�����������������ᣨH2C2O4���������������Ʒ�Ӧ����CaC2O4������
��5������ͭ��ĩ��ˮ����ɫ��������ƾ��壨CaC2O4xH2O����Ʒ���¼��ȣ�B�й۲쵽����ͭ��ĩ��������˵����Ӧ������ˮ��
��6��C�г���ʯ��ˮ������ǣ�˵����Ӧ������CO2���壻G�г���ʯ��ˮ������ǣ�˵����Ӧ������CO���壬��Ϊһ����̼������ͭ��Ӧ�����ɶ�����̼��
��7����������ʵ�������֪��������ж�����̼���ɣ�����������������Һ������ʹ������̼��ȫ���գ�G�г���ʯ��ˮҲ�����ǣ�����D �� E װ֮��������һ��ʢ����ʯ��ˮ��ϴ��ƿ������ϴ��ƿ�г���ʯ��ˮ������ǣ���G�г���ʯ��ˮ�������˵������ֽ�����˶�����̼����ͬѧ��Ϊ�������۲��Ͻ��������ܵó���һ����̼���ɵĽ��ۡ����ɿ�����������û�н�������̼��ȫ���ա�
��8�����¶�Ϊ200������ʱ������ȫ��ʧȥ�ᾧˮ�������нᾧˮ������Ϊ14.6g-12.8g=1.8g��
�ڸ���ͼ���֪0��200���Ǿ���ʧȥ�ᾧˮ�Ĺ��̣�14.6��CaC2O4xH2Oʧȥˮ������12.8��CaC2O4��
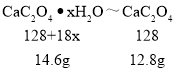
![]() �����x�T1��
�����x�T1��
��800������ʱ���������������Ϊ��������������غ㶨�ɿ�֪���ù����������������ƣ������������Ƶ�����Ϊy��
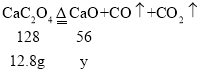
![]() �����x=5.6g����ͼ��m��ֵ��5.6g��
�����x=5.6g����ͼ��m��ֵ��5.6g��
�ܸ���ͼ���֪800������ʱ����Ӧ�����������ƣ������ƿ�������̼��Ʒֽ����ã�400��ʱ����Ʒֽ⣬����������̼��ƣ�������̼��ƣ���10g�Ĺ���Ϊ̼��ƣ�10g̼��ƹ����У���Ԫ�ص�����=![]() ��̼Ԫ�ص�����=
��̼Ԫ�ص�����=![]() ����Ԫ�ص�����=10g-4g-1.2g=4.8g��12.8g������и�Ԫ�ص�����=
����Ԫ�ص�����=10g-4g-1.2g=4.8g��12.8g������и�Ԫ�ص�����=![]() ��̼Ԫ�ص�����=
��̼Ԫ�ص�����=![]() ����Ԫ�ص�����=12.8g-2.4g-4g=6.4g�����������غ㶨�ɿ�֪������Ʒֽ��IJ����г���̼����⣬����������̼Ԫ�ص�����=2.4g-1.2g=1.2g����Ԫ�ص�����=6.4g-4.8g=1.6g���������ʵ�����=1.2g+1.6g=2.8g���������������������ʵ�����=12.8g-10g=2.8g��ͬ�����������̼ԭ������ԭ�ӵĸ�����=
����Ԫ�ص�����=12.8g-2.4g-4g=6.4g�����������غ㶨�ɿ�֪������Ʒֽ��IJ����г���̼����⣬����������̼Ԫ�ص�����=2.4g-1.2g=1.2g����Ԫ�ص�����=6.4g-4.8g=1.6g���������ʵ�����=1.2g+1.6g=2.8g���������������������ʵ�����=12.8g-10g=2.8g��ͬ�����������̼ԭ������ԭ�ӵĸ�����=![]() �����Բ���Ʒֽ��IJ����г���̼����⣬����CO����Ӧ�Ļ�ѧ����ʽ�ǣ�CaC2O4
�����Բ���Ʒֽ��IJ����г���̼����⣬����CO����Ӧ�Ļ�ѧ����ʽ�ǣ�CaC2O4![]() CaCO3+CO����
CaCO3+CO����

 ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д� ͬ����ϰ����ʦ����ѧ������ϵ�д�
ͬ����ϰ����ʦ����ѧ������ϵ�д� ����ϰ�⽭��ϵ�д�
����ϰ�⽭��ϵ�д� ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�����Ŀ�����������쵪�ʣ����ء�̼淋ȣ������Ϸ��ϡ����ᡢ����ȣ��㷺Ӧ���ڻ������Ṥ��������ҩ���ϳ���ά������
��ҵ�ư����Թ�����ͨ�������������ڸ��¸�ѹ�ʹ����������»������ɵģ����������������ǡ����ȫ��Ӧʱ��������________��
��Ŀǰ��һ�����˹��̵������·�����Ӧ����ʾ��ͼ���£�
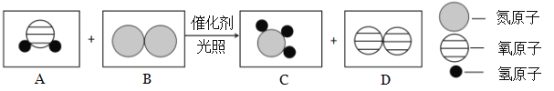
��1�����ݷ�Ӧ����ʾ��ͼд����ѧ����ʽ��_____________��
��2�������������������ˮ���ã������γ�����������д�������м�Ļ�ѧʽ����������е�Ԫ�صĻ��ϼۣ�_________��
���ǵ��ʵ���Ҫԭ�ϡ�ij���ʳ������̬����(NH4)2SO4�Ĺ����������£�
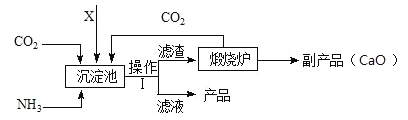
��1���������еIJ�����������_________��
��2�������XӦΪ_____������ĸ��
A H2SO4 B CaSO4 C SO2
������Ҳ�ǹ�ҵ��ά�����ƴ������Ҫԭ�ϡ�
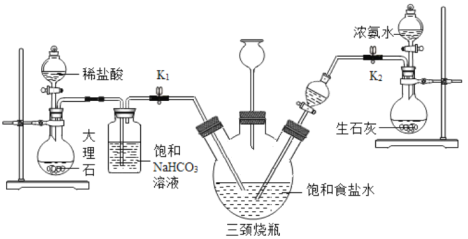
ij��ȤС���������װ��ģ����ά�����Ʊ�̼�����ƣ�ʵ��������£�
a���ر�K1����K2ͨ��NH3�������������ʣ������ȶ���K1ͨ��CO2��
b����������ƿ�ڳ��ֽ϶�NaHCO3����ʱ���ر�K2ֹͣͨNH3��һ��ʱ��ر�K1ֹͣͨCO2��
c����������ƿ�ڵķ�Ӧ�������ˡ�ϴ�ӡ����¸���������ù������ڳ��������м��ȣ���¼ʣ�����������
����ʱ��/min | t0 | t1 | t2 | t3 | t4 | t5 |
ʣ���������/g | δ��¼ | 30.6 | 27.4 | 23.8 | 21.2 | 21.2 |
�����ϣ����³�ѹ�£�1���ˮԼ���ܽ�700���������1���ˮԼ���ܽ�1���������̼��
��ش��������⣺
��1��������ƿ�����ɵ���һ������һ���̬���ʣ�д�����з����Ļ�ѧ��Ӧ����ʽ��__________�����ȹ��˵õ���NaHCO3��������Ӧ��2NaHCO3 ![]() Na2CO3 + CO2��+ H2O��
Na2CO3 + CO2��+ H2O��
��2��������ƿ�����ӵij���©������Ҫ������__________��
��3����K2ͨ��NH3һ��ʱ��Ŵ�K1ͨ��CO2��ԭ����__________��
��4������̼��������Һ��������______________��
��5������ʵ���¼������t2 minʱNaHCO3����ķֽ��ʣ��ѷֽ��NaHCO3���������ǰԭNaHCO3�����ı�ֵ������д���������__________��
����Ŀ����һ�ܱ������У����������ʣ���һ�������´���ij����Ӧ����÷�Ӧǰ������ʵ��������±�����֪X����Է�������Ϊn��Q����Է�������Ϊ2n.������������ȷ���� ( )
���� | X | Y | Z | Q |
��Ӧǰ����/g | 4 | 10 | 1 | 21 |
��Ӧ������/g | 0 | 12 | 15 | ���� |
A. �÷�Ӧ�����Q����Ϊ12g
B. ��Ӧ������15gZ
C. �û�ѧ����ʽ��X��Q�Ļ�ѧ������֮��Ϊ2��3
D. �÷�Ӧ��Y��Q����֮��Ϊ1��1