��Ŀ����
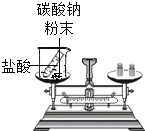
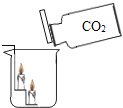
ijʵ��С�齫�����⻯�ƣ�CaH2������ˮ���γ�����ɫ������Һ���������м���̼������Һ���г��������������˺�õ���������Һ��Ȼ�������������֤����Һ���ʳɷֵ�̽����
��1�����������ϣ��⻯���ڳ���������ˮ��Ӧ�����������ƺ�������д���÷�Ӧ�Ļ�ѧ����ʽ______________��
��2����������֤������ֻ����̼��ƣ���������֤�������Ļ�ѧ����ʽΪ______________��
��3����������⣩��Һ�����ʵijɷ���ʲô��
��4����������裩����һ��NaOH
�������NaOH��Na2CO3
��������NaOH��________��
�����ģ�NaOH��Na2CO3��Ca��OH��2
��5�������۷���������������Ϊ��������Dz���____��ԭ����______________��
��6����ʵ������ۣ�
ʵ�鲽�� | ���� | ���� |
����I��ȡ������Һ�������м�������������Һ | ________ | ����������� |
�������ȡ������Һ�������е�������Na2CO3��Һ | ������ɫ���� | ����____���� |
��7������չ���죩�ڷ�����Ӧ���������ʵijɷ�ʱ���������������⣬���迼��___________��
 ��ʦ����ָ���ο�ʱϵ�д�
��ʦ����ָ���ο�ʱϵ�д������й�����֪ʶ�Ĺ�����ȫ��ȷ��һ����
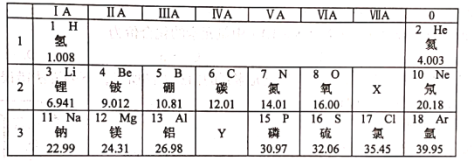
A��������; | B��Ԫ��֮�� |
Һ��������ҽ�� ��������������ɱ�� | ��ˮ�к�������Ԫ�أ���Ԫ�� �����к�������Ԫ�أ���Ԫ�� |
C�����ʷ��� | D������֪ʶ |
������ˮ���������� ��ȥ�����еĿ��������ʣ����ó���ת���� | �����¶�ЧӦ��ֲ�����ֽ��ܼ��� ����ˮ����Ⱦ����ֹʹ��ϴ�Ӽ� |
A.A B.B C.C D.D