��Ŀ����
�ס��������о���ѧϰС��ֱ��Ʊ������������ð��������ʣ����������ȵ�����ͭ��Ӧ������ͭ��ˮ�͵���������֤�����е�����ԭ�Ӹ����ȣ����������ʵ�����̣�
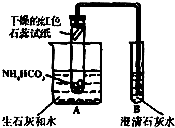
ʵ���У������Ƶõİ����ž�ϴ��ƿǰ����װ���еĿ�����������ϴ��ƿ�������ռ�װ�ã�������������ͭ����Ӧ��ɺ�ɫ������ͭת��Ϊ��ɫ��ͭ��
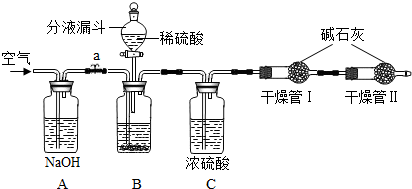
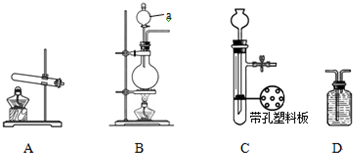
��ͼA��B��CΪ�ס�����С���Ʊ�����ʱ�����õ���װ�ã�DΪʢ������Ũ�����ϴ��ƿ��
��С���ã���Ӧǰ����ͭ������Ϊm1g������ͭ��Ӧ��ת���ɵ�ͭ������Ϊm2g�����ɵĵ����ڱ�״���µ����V1L����֪��״���£�22.4L������������28g����
��С���ã�ϴ��ǰװ��D������m3g��ϴ����װ�ú�D������m4g�����ɵĵ����ڱ�״���µ����V2L��
��ش��������⣺
��1��д������a�����ƣ�______
��2�����Aװ�������Եľ������������______��
��3���ס�����С��ѡ���˲�ͬ�����Ʊ��������뽫ʵ��װ�õ���ĸ��ź��Ʊ�ԭ����д���±��ո��У�
��4����С�����������ݼ�����������е������ԭ�Ӹ���֮��Ϊ______��
��5����С�����������ݼ�����������е������ԭ�Ӹ�����С������ֵ����ԭ����______��Ϊ�ˣ���С����ԭ��ʵ�����̵Ļ����ϣ���______��λ��������һ��װ������ҩƷ��ʵ������������ʵ�飮����ʵ��ǰ���ҩƷ�������仯�����ɵ����������Ҳ�ó��˺�����ʵ��������ҩƷ��������______��

ʵ���У������Ƶõİ����ž�ϴ��ƿǰ����װ���еĿ�����������ϴ��ƿ�������ռ�װ�ã�������������ͭ����Ӧ��ɺ�ɫ������ͭת��Ϊ��ɫ��ͭ��
��ͼA��B��CΪ�ס�����С���Ʊ�����ʱ�����õ���װ�ã�DΪʢ������Ũ�����ϴ��ƿ��

��С���ã���Ӧǰ����ͭ������Ϊm1g������ͭ��Ӧ��ת���ɵ�ͭ������Ϊm2g�����ɵĵ����ڱ�״���µ����V1L����֪��״���£�22.4L������������28g����
��С���ã�ϴ��ǰװ��D������m3g��ϴ����װ�ú�D������m4g�����ɵĵ����ڱ�״���µ����V2L��
��ش��������⣺
��1��д������a�����ƣ�______
��2�����Aװ�������Եľ������������______��
��3���ס�����С��ѡ���˲�ͬ�����Ʊ��������뽫ʵ��װ�õ���ĸ��ź��Ʊ�ԭ����д���±��ո��У�
| ʵ��װ�� | ʵ��ҩƷ | �Ʊ�ԭ�� | |
| ��С�� | A | �������ơ������ | ��Ӧ�Ļ�ѧ����ʽΪ ��______ |
| ��С�� | ��______ | Ũ��ˮ���������� |
��5����С�����������ݼ�����������е������ԭ�Ӹ�����С������ֵ����ԭ����______��Ϊ�ˣ���С����ԭ��ʵ�����̵Ļ����ϣ���______��λ��������һ��װ������ҩƷ��ʵ������������ʵ�飮����ʵ��ǰ���ҩƷ�������仯�����ɵ����������Ҳ�ó��˺�����ʵ��������ҩƷ��������______��
��1������a������Ϊ ��Һ©��
��2�����Aװ�������Եľ���������������ӵ��ܣ������ܲ���ˮ�У����ֽ����Թܣ����ܿ������ݲ������ɿ��ֺ�������ˮ�������γ�һ���ȶ���ˮ��
��3������������������立�Ӧ��������ơ�������ˮ����Ӧ�Ļ�ѧ����ʽΪ��NH4��2SO4+Ca��OH��2�T2NH3��+2H2O+CaSO4
��Ũ��ˮ��Һ�壬���������ǹ��壬Ϊʹ�����ݳ������ü��Ƚ��Ͱ������ܽ�ȣ���ѡBװ��
��4����Ӧǰ����ͭ������Ϊm1g������ͭ��Ӧ��ת���ɵ�ͭ������Ϊm2g��������ͭ����Ԫ�ص�����Ϊm1-m2�����ɵ�ˮ����Ԫ�ص�������������ͭ��Ԫ�ص������������ɵ�ˮ����ԭ�ӵĸ���Ϊ
��ˮ����ԭ�Ӹ�������ԭ�Ӹ����������������ԭ�Ӹ���Ϊ
��2�����ɵĵ����ڱ�״���µ����V1L�������е�ԭ�ӵĸ���Ϊ
����˵���ԭ�Ӹ�����Ϊ
��
��2=5V1��7��m1-m2����
��5����С�����������ݼ�����������е������ԭ�Ӹ�����С������ֵ����ԭ����ϴ��ƿD�е�Ũ����������˷�Ӧ���ɵ�ˮ����������δ��Ӧ�İ������Ӷ�ʹ����������ƫ�ߣ������ ϴ��ƿDǰ��λ��Ӧ������һ��װ�м�ʯ�ң���ˮ����ͭ���������ơ������Ƶȣ���ʵ������ֻ����ˮ����С���
�ʴ�Ϊ��
��1����Һ©��
��2�����ӵ��ܣ������ܲ���ˮ�У����ֽ����Թܣ����ܿ������ݲ������ɿ��ֺ�������ˮ�������γ�һ���ȶ���ˮ��
��3���٣�NH4��2SO4+Ca��OH��2�T2NH3��+2H2O+CaSO4��B
��4��5V1��7��m1-m2����
��5��ϴ��ƿD�е�Ũ����������˷�Ӧ���ɵ�ˮ����������δ��Ӧ�İ������Ӷ�ʹ����������ƫ�ߡ�ϴ��ƿDǰ
��ʯ�ң�����ˮ����ͭ���������ơ������Ƶȣ�
��2�����Aװ�������Եľ���������������ӵ��ܣ������ܲ���ˮ�У����ֽ����Թܣ����ܿ������ݲ������ɿ��ֺ�������ˮ�������γ�һ���ȶ���ˮ��
��3������������������立�Ӧ��������ơ�������ˮ����Ӧ�Ļ�ѧ����ʽΪ��NH4��2SO4+Ca��OH��2�T2NH3��+2H2O+CaSO4
��Ũ��ˮ��Һ�壬���������ǹ��壬Ϊʹ�����ݳ������ü��Ƚ��Ͱ������ܽ�ȣ���ѡBװ��
��4����Ӧǰ����ͭ������Ϊm1g������ͭ��Ӧ��ת���ɵ�ͭ������Ϊm2g��������ͭ����Ԫ�ص�����Ϊm1-m2�����ɵ�ˮ����Ԫ�ص�������������ͭ��Ԫ�ص������������ɵ�ˮ����ԭ�ӵĸ���Ϊ
| m1-m2 |
| 16 |
| m1-m2 |
| 16 |
| V1��2 |
| 22.4 |
| V1��2 |
| 22.4 |
| m1-m2 |
| 16 |
��5����С�����������ݼ�����������е������ԭ�Ӹ�����С������ֵ����ԭ����ϴ��ƿD�е�Ũ����������˷�Ӧ���ɵ�ˮ����������δ��Ӧ�İ������Ӷ�ʹ����������ƫ�ߣ������ ϴ��ƿDǰ��λ��Ӧ������һ��װ�м�ʯ�ң���ˮ����ͭ���������ơ������Ƶȣ���ʵ������ֻ����ˮ����С���
�ʴ�Ϊ��
��1����Һ©��
��2�����ӵ��ܣ������ܲ���ˮ�У����ֽ����Թܣ����ܿ������ݲ������ɿ��ֺ�������ˮ�������γ�һ���ȶ���ˮ��
��3���٣�NH4��2SO4+Ca��OH��2�T2NH3��+2H2O+CaSO4��B
��4��5V1��7��m1-m2����
��5��ϴ��ƿD�е�Ũ����������˷�Ӧ���ɵ�ˮ����������δ��Ӧ�İ������Ӷ�ʹ����������ƫ�ߡ�ϴ��ƿDǰ
��ʯ�ң�����ˮ����ͭ���������ơ������Ƶȣ�

��ϰ��ϵ�д�
 ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д�
�����Ŀ