��Ŀ����
����Ŀ���ҹ�������ѧ�Һ�°�������Ϊ���紿�ҵ�����ķ�չ�����˽ܳ��������÷���NaCl��NH3��CO2��Ϊԭ�������Ƶ�NaHCO3�������������������йػ�ѧ��ӦΪ��
NH3+CO2+H2O�TNH4HCO3
NH4HCO3+NaCl�TNaHCO3��+NH4Cl
2NaHCO3�T�TNa2CO3+CO2��+H2O
����(1)̼������뱥��ʳ��ˮ��Ӧ��������̼�����ƾ����ԭ����_____��
a��NaHCO3������ˮ
b��NH4Cl������ˮ
c��NaHCO3���ܽ����Խ�С������Һ�����Ƚᾧ����
d��NH4Cl���ܽ����Խ�С������Һ�����Ƚᾧ����
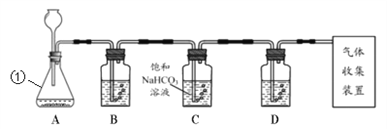
(2)ij�С���������ԭ����ģ��̼�����Ƶ��Ʊ�ʵ�飺��������̼����ͨ�백���ı���ʳ��ˮ���Ʊ�̼�����ƣ�ʵ��װ������ͼ��ʾ��
˵����(1)ͼ�мг֡��̶��õ�����δ����
(2)�������ı���ʳ��ˮ��������ʳ��ˮ��ͨ�백��������
��ش�
����ͼװ������һ����������Ӧ��θ���_____________���С���������װ�ú����Լ�����ʵ�顣
��ʵ����������NaHCO3����IJ�����_____(�����������)��
��ʵ��ʱ��Ϊ��߶�����̼��������������ʳ��ˮ����ͨ��NH3�����͵�ԭ����______��
�����÷����õĴ����г����������Ȼ�����������ͼ��ʾװ�����ⶨ������Ʒ��̼���Ƶ���������(ͼ�мг֡��̶��õ�����δ����)��
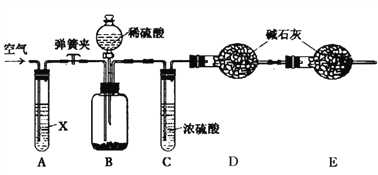
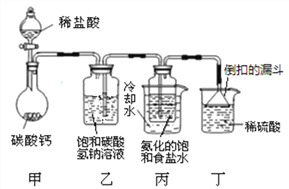
ʵ�鲽�����£�
�ٰ���ͼ����װ��������������ԣ�
��ȷ�Ƶ�ʢ�м�ʯ��(�������ƺ������ƵĻ����)�ĸ����D������Ϊ83.4g��
��ȷ�Ƶ�6.0g������Ʒ����װ��B�Ĺ��ƿ�У�
�ܴ�װ��B�ķ�Һ©����������������ϡ�����������ٲ�������Ϊֹ��
�ݴ��ɼУ����Թ�A�л���������������ӣ�Ȼ��Ƶø����D��������Ϊ85.6g��
��ش�
(1)���������Ŀ����____________��װ��A���Լ�X������������ѡ��______(�ѧʽ)��
(2)��û��Cװ�ã���ᵼ�²ⶨ���_____(����ƫ��������ƫС��������Ӱ����)��
(3)����ʵ���в�õ��й����ݣ�������Ʒ��̼���Ƶ���������Ϊ_____(�ðٷ�����ʾ���������һλС��)��
���𰸡� c ��װ���еĵ���Ӧ��Ϊ�����̳� ���� NH3��ˮ�е��ܽ��Զ����CO2 ����װ���е�������ʹ��Ӧ���ɵĶ�����̼������ȫ��D�м�ʯ������ NaOH ƫ�� 88.3%
��������������ѧ֪ʶ��������Ϣ֪����( 1 )̼������뱥��ʳ��ˮ��Ӧ��������̼�����ƾ����ԭ����NaHCO3���ܽ����Խ�С������Һ�����Ƚᾧ������( 2 ) ��������̼����ͨ�백���ı���ʳ��ˮ���Ʊ�̼�����ơ�����ͼװ������һ����������Ӧ����Ϊ��װ���еĵ���Ӧ��Ϊ�����̳����������ܴﵽϴ����Ŀ�ģ��С���������װ�ú����Լ�����ʵ�顣��ʵ���������� NaHCO3����IJ����ǹ��ˣ������ǰѲ�����Һ��Ĺ���������Һ������һ�ַ�������ʵ��ʱ��Ϊ��߶�����̼��������������ʳ��ˮ����ͨ��NH3�����͵�ԭ����NH3��ˮ�е��ܽ��Զ����CO2���÷����õĴ����г����������Ȼ��ƣ��ֲⶨ������Ʒ��̼���Ƶ�����������(1)���������Ŀ��������װ���е����壬ʹ��Ӧ���ɵĶ�����̼������ȫ��D�м�ʯ�����ա�װ�� A ���Լ� X ������������ѡ��NaOH��NaOH�������̼��Ӧ��(2)��û�� C װ�ã���ᵼ�²ⶨ���ƫ��ˮ������������ܣ�ʹ��ö�����̼�������ӡ�(3)����ʵ���в�õ��й����ݣ�������Ʒ��̼���Ƶ���������Ϊ�����ɶ�����̼������85.6g -83.4g��2.2g���μӷ�Ӧ��̼����������Na2 CO3��CO2��106��44�� ![]() ��
��![]() ��x��5.3g��
��x��5.3g�� ![]() ��100����88.3%��
��100����88.3%��
�㾦����Ϊϴ��װ��Ӧ�dz����̳���