��Ŀ����
��Ԫ������������Ԫ�أ������ʳ������ȡ���㣬ҽ�����顰���ơ���
��1��ҽ�����顰���ơ���ָ������ �����Ԫ�ء����ʡ���ԭ�ӡ���
��2��̼�����̼Ԫ�صĻ��ϼ�Ϊ�� ����
��3��Ϊ�ⶨ�ò��Ƽ���̼��Ƶ������������ֳ�ȡ15�˵���Ʒ�����ձ�������м���������ϡ�����ַ�Ӧ�������ɷֲ�����Ԫ�ء�������ˮҲ����ϡ���ᷴӦ������������Ϊ4.4g��
���ж���ȡ��Ʒ����ȫ��Ӧ������Ϊ�� ����
�ڸò��Ƽ���Ʒ��̼��Ƶ���������Ϊ���٣������ü����������ʾ������������һλС����
��1��ҽ�����顰���ơ���ָ������ �����Ԫ�ء����ʡ���ԭ�ӡ���
��2��̼�����̼Ԫ�صĻ��ϼ�Ϊ�� ����
��3��Ϊ�ⶨ�ò��Ƽ���̼��Ƶ������������ֳ�ȡ15�˵���Ʒ�����ձ�������м���������ϡ�����ַ�Ӧ�������ɷֲ�����Ԫ�ء�������ˮҲ����ϡ���ᷴӦ������������Ϊ4.4g��
���ж���ȡ��Ʒ����ȫ��Ӧ������Ϊ�� ����
�ڸò��Ƽ���Ʒ��̼��Ƶ���������Ϊ���٣������ü����������ʾ������������һλС����
��1��Ԫ�� ��2�� +4������3���������ݲ�����������������66.7%
�����������1��ҽ�����顰���ơ���ָ����Ԫ�أ�2��̼�����̼Ԫ�صĻ��ϼۣ����ݻ�������Ԫ�صĻ��ϼ۵Ĵ����Ͳ�Ϊ�㣬�ʿ�ȷ��̼Ԫ�صĻ��ϼ�Ϊ+4�ۣ���3�����ж���ȡ��Ʒ����ȫ��Ӧ������Ϊ�����ݲ�����������������
����μӷ�Ӧ��̼��Ƶ�����Ϊx
CaCO3 + 2HCl = CaCl2 + CO2��+ H2O
100 44
X 4.4g
100��44=X��4.4g
X=10g
�ò��Ƽ���Ʒ��̼��Ƶ���������Ϊ10 g /15 g��100%="66.7%"
�𣺸ò��Ƽ���Ʒ��̼��Ƶ���������Ϊ66.7%

��ϰ��ϵ�д�
 �ܿ�����ĩ��̾�ϵ�д�
�ܿ�����ĩ��̾�ϵ�д�
�����Ŀ
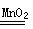 2H2O+O2��)
2H2O+O2��)