��Ŀ����
����Ŀ��ijƷ�ƴ����к����������Ȼ��ơ�ij��ѧ̽��С�����ⶨ��Ʒ�ƴ���Ĵ��ȣ���̼���Ƶ�������������
��һ������ͬѧ�����ͼ��ʾʵ�飺
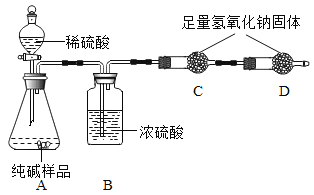
��ʵ�鲽�裩
�ٰ�װ��ͼ���������������_________
�ڳ�ȡ13.0g��Ʒ������ƿ�У�������������ˮ�ܽ⣬��������װ���м�����Ӧ��ҩƷ��
�۳���װ��C������
�ܴ�Һ©����������ϡ���ᣬֱ��__________________����ʵ������Ϊֹ��
���ٴγ���װ��C��������
�������Ʒ��̼���Ƶ���������
��ʵ�������
��1��Aװ���з�Ӧ�Ļ�ѧ����ʽΪ_________
��2��Bװ�õ�������_________
��3�����û��Bװ�ã���������Ʒ��̼���Ƶ���������__________������ƫ��������ƫС��������������
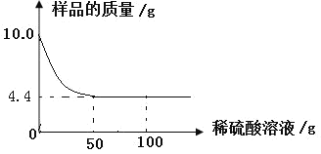
��4���Ƶô�����Ʒ������Ϊ13.0g��ʵ��ǰ����Cװ�ã�����ҩƷ���������ֱ�Ϊ61.2g��65.6g����ô�����Ʒ�Ĵ���Ϊ________________����ȷ��0.1%��
��5�������������ʵ�飬����˵���������__________������ţ�
A.���ó���©�������Һ©�� B.����ϡ�������ϡ����
C.��Cװ���Ҷ˷�ס���ɲ�ҪDװ�� D.ʵ����Ӧ�����μ�ϡ����
E.Dװ�õ������Ƿ�ֹ�����еĶ�����̼��ˮ��������Cװ���У����ʵ����
����������ͬѧ�������ɳ����ķ������ⶨ��Ʒ�д�����������������������ʵ�飺
��1���жϼ����Ȼ�����Һ�Ƿ�����ĺ��ʷ�����_________������ţ���Ȼ��۲������ж�
A.���û����X�������м����μ��Ȼ�����Һ�����ް�ɫ�������֣����Ȼ����ѹ���
B.������X�м����μ�ϡ���ᣬ���а�ɫ�������ɣ����Ȼ����ѹ�����
��2���ж������Ƿ�ϴ�ɾ������Բ�ȡ������ϴ��Һ�еμ�__________������ţ���Ȼ��۲������ж�
A.�Ȼ�����Һ B.ϡ���� C.��������Һ D.ϡ����
��3������ʵ�����ݣ���������Ʒ��̼���Ƶ���������Ϊ88.3%��
��ʵ�鷴˼��
�ס�������ͬѧ�����Ʒ����̼���Ƶ���������������Ϊ_______�飨��������������������ȷ����һ����ڽϴ�ƫ���ԭ�������_________
���𰸡�װ�������� ���������� Na2CO3+H2SO4�TNa2SO4+H2O+CO2�� ����ˮ���� ƫ�� 81.5% ABC A C �� ��Ӧ���ɵĶ�����̼���ܱ�����������ȫ����
��������
̼���ƺ�ϡ���ᷴӦ���������ơ�ˮ�Ͷ�����̼����ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼���������ܺ������ӽ�����ɰ�ɫ�����Ȼ�����
��һ��[ʵ�鲽��]�ٰ�װ��ͼ���������������װ�������ԣ��ڳ�ȡ13.0g��Ʒ������ƿ�У�������������ˮ�ܽ⣬��������װ���м�����Ӧ��ҩƷ���۳���װ��C���������ܴ�Һ©����������ϡ���ᣬֱ������������Ϊֹ�����ٴγ���װ��C�����������������Ʒ��̼���Ƶ�����������
[ʵ�����]��1��Aװ���У�̼���ƺ�ϡ���ᷴӦ���������ơ�ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+H2SO4�TNa2SO4+H2O+CO2����
��2��Bװ�õ�����������ˮ�������Է�ֹˮ�������������ƹ������գ�Ӱ��ʵ������
��3�����û��Bװ�ã���ˮ�����ᱻ�����������գ��Ӷ�����������Ʒ��̼���Ƶ���������ƫ��
��4����Ӧ���ɶ�����̼����Ϊ��65.6g-61.2g=4.4g����̼��������Ϊx��
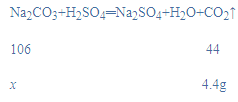
![]()
x=10.6g��
��ô�����Ʒ�Ĵ���Ϊ��![]() ��100%=81.5%��
��100%=81.5%��
��5��A������©�����ܿ���Һ����������ѡ��˵������ȷ��
B��������лӷ��ԣ�HCl���ڶ�����̼�У���Cװ�����պ��Ӱ�촿�ȵIJⶨ����ѡ��˵������ȷ��
C�����ܽ�Cװ���Ҷ˷�ס��ȥ��Dװ�ã�������Ϊ�����Cװ���Ҷ˷�ס��ȥ��Dװ�ã��ᵼ�¶�����̼���ܱ�����������ȫ���գ�Ӱ��ʵ��������ѡ��˵������ȷ��
D��ʵ����Ӧ�����μ�ϡ���ᣬĿ����ʹ�����Ķ�����̼��ȫ�������������գ���ѡ��˵����ȷ��
E��Dװ�õ������Ƿ�ֹ�����еĶ�����̼��ˮ��������Cװ���У����ʵ������ѡ��˵����ȷ��
��������1���жϼ����Ȼ�����Һ�Ƿ�����ĺ��ʷ����Ǿ��û����X�������м����μ��Ȼ�����Һ�����ް�ɫ�������֣����Ȼ����ѹ�����
��2���ж������Ƿ�ϴ�ɾ������Բ�ȡ������ϴ��Һ�еμ���������Һ�����������������˵��ϴ�Ӹɾ���
[ʵ�鷴˼]�ס�������ͬѧ�����Ʒ����̼���Ƶ������������Ҹ�ȷ����һ����ڽϴ�ƫ���ԭ������Ƿ�Ӧ���ɵĶ�����̼���ܱ�����������ȫ���ա�

 ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д� ͬ����ϰ����ʦ����ѧ������ϵ�д�
ͬ����ϰ����ʦ����ѧ������ϵ�д� ����ϰ�⽭��ϵ�д�
����ϰ�⽭��ϵ�д� ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�����Ŀ�����±��еļ���������μ�����������Һ����������Ӧ������������������������ҵ�������ϵ��������������������
ѡ�� | �� | �� |
A. | ͭ��п�Ļ�����ĩ | ϡ���� |
B. | �����ϡ����Ļ����Һ | �Ȼ�����Һ |
C. | �Ȼ��ƺ��Ȼ��ƵĻ����Һ | ̼������Һ |
D. | ������Ȼ�ͭ�Ļ����Һ | ����������Һ |
A. AB. BC. CD. D