��Ŀ����
����Ŀ����ѧ���������ϢϢ��أ������û�ѧ֪ʶ�ش��������⡣
��1��ʪ�·��������±����������ɵÿ죬������Ϊ_____________________________��
��2�������Ҵ������У��Ҵ��������ǣ��û�ѧ����ʽ��ʾ��_________________________��
��3��ҽ���ϡ����͡�����Ҫ�ɷ������ᱵ����������̼�ᱵ������ᱵ��ԭ���ǣ��û�ѧ����ʽ��ʾ��______________________________��
��4��������������������ͭ�ķ�Һ�м���ij�ֽ������ɻ�������������ͭ���÷�Ӧ�Ļ�ѧ����ʽ��____________________________________��
��5����ת��������Ч����β���е�CO��NO��NO2��̼�⻯������к�������������ŷš���ת������ͨ�����ò��ȹ��ؽ�������������д��CO��NO�ڴ����������·�����Ӧ����CO2��N2�Ļ�ѧ����ʽ___________________��
���𰸡��������¶ȸߣ��¶����ߣ����ӵ��˶����ʼӿ� ![]() BaCO3+2HCl=BaCl2+H2O+CO2�� Fe+CuSO4=Cu+FeSO4
BaCO3+2HCl=BaCl2+H2O+CO2�� Fe+CuSO4=Cu+FeSO4 ![]()
��������
��1��ʪ�·��������±����������ɵÿ죬����Ϊ�������¶ȸߣ��¶����ߣ����ӵ��˶����ʼӿ죻
��2�������Ҵ������У��Ҵ�ȼ�����ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ��![]()
��3�������Ա��ζ������к���̼�ᱵ����θҺ�е����ᷴӦ�������Ȼ�����ˮ�Ͷ�����̼��������̼�ᱵ������ᱵ����ѧ����ʽΪ��BaCO3+2HCl=BaCl2+H2O+CO2����
��4����������ͭ��Һ��Ӧ��������������ͭ����Ӧ�Ļ�ѧ����ʽΪFe+CuSO4=Cu+FeSO4��
��5��CO��NO�ڴ����������·�����Ӧ����CO2��N2����Ӧ�Ļ�ѧ����ʽ�ǣ�![]() ��
��

����Ŀ�����л�ѧ������3��Ԫ��X��Y��Z�����Ϣ���±���ʾ
Ԫ�� | X | Y | Z |
�����Ϣ | ���ĵ��ʼ���һ�������Դ | �ؿ��к�������Ԫ�� | ����һ�ֵ���������Ȼ���ڵ���Ӳ������ |
��1���Ļ�ѧʽ��______���ҵ�������______
��2��ZY2����Z�ĵ����ڸ��������·�Ӧ����ZY���÷�Ӧ�Ļ�ѧ����ʽ��____________
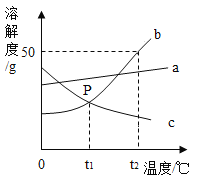
��3��A��B��C��������3��Ԫ���е�һ�ֻ�������ɵĵ��ʻ��G��Ca��Y��Z����Ԫ����ɡ�����֮���ת����ϵ����ͼ��ʾ����������ʾת����ϵ�����ַ�Ӧ������P��Ӧ��������ȥ��
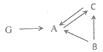
��G�Ļ�ѧʽ��________
����A�в���XԪ�أ���B�Ļ�ѧʽΪ______C��A�Ļ�ѧ����ʽ��________
����A�в���ZԪ�أ�B��C�Ļ�ѧ����ʽ��_________