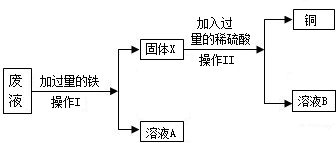
��Ŀ����
��ȤС��ͬѧ��ʵ���Ҵ�����Һʱ����һƿ�Ѿ��н�����ʯ��ȡ�������뺬������ķ�Һ�У����ַ�Һ�в����������ݣ�ͬѧ��Ϊ��һ���˽���ƿ��ʯ�ұ��ʵij̶ȣ���������̽���������Э����ɣ�
��̽���һ���ⶨ���ʵ���ʯ����Ʒ��̼��Ƶ���������
����ʦ��ָ����ͬѧ���������ͼ��ʾʵ��װ�ã�����̨��ȥ������ʵ�飬��ȡһ��������Ʒ��ͨ���ⶨ��Ʒ��ϡ���ᷴӦ���������������������̼��Ƶ�����������װ�����������ã���������Ļӷ�����ÿ����Ӧ�����ö�����ȫ�ģ���
�������ϣ�Ũ������к�ǿ����ˮ�ԣ���ʯ�ҳ���������ˮ�����Ͷ�����̼��
��1������������ϡ���ᣬװ��A����Ʒ��������Ļ�ѧ����ʽΪ������
��2��ʵ������У�װ��B��������������װ��C�з�����Ӧ�Ļ�ѧ����ʽΪ������ͨ���ⶨװ��C��ʵ��ǰ��������������ӵ�������Ϊ��Ʒ��ϡ���ᷢ����Ӧ�����������������
��3����װ��A�м�������ǰ��������k���ȶ�װ��A��B�������ӣ�ͨ���ѳ�ȥCO2�Ŀ���һ��������ž�װ��A��B����CO2�Ŀ������ٽ���װ��C��D������ϡ���ᣬ��װ��A�еķ�Ӧ����������һ�δ�����k��������װ��ͨ���ѳ�ȥCO2�Ŀ���һ���������ʵ����������ƫС��������Ϊ��������ʵ��ⶨ�ñ��ʵ���ʯ����Ʒ��̼��Ƶ���������Ϊ65%����û��װ��D������ʹ�ⶨ���������ѡ�����65%����С��65%������
��4��ͬѧ����Ϊ����ƿ���ʵ���ʯ���Կ��������Ƴ����ʯ��ˮ�����������������
| ��1������ϡ������̼��Ƶķ�Ӧд����Ӧ�ķ���ʽ�� ��2������Ũ��������ˮ�ԣ�����������Һ�����ն�����̼�����ش� ��3������A��Bװ���ڵĶ�����̼δ���������Ʒ�Ӧ����ʵ����������ƫС��ԭ���ݿ����еĶ�����̼�����������Ʒ�Ӧ��������û��װ��D����Բⶨ�����Ӱ�죻 ��4������̼���������ˮ������ | |
| ��� | �⣺��1����װ��A�У������ϡ��������̼��Ʒ�Ӧ����Ӧ�ķ���ʽ�ǣ�CaCO3+2HCl=CaCl2+H2O+CO2���� ��2������Ũ��������ˮ�ԣ����ԣ�װ��B�������Ǹ��������̼���壻���������������������̼��Ӧ�����ԣ�װ��C�з�����Ӧ�Ļ�ѧ����ʽΪ��CO2+2NaOH═Na2CO3+H2O��ͨ���ⶨװ��C��ʵ��ǰ��������������ӵ�������Ϊ��Ʒ��ϡ���ᷢ����Ӧ�Ķ�����̼����������� ��3���������֪������������װ��ͨ���ѳ�ȥCO2�Ŀ���һ�����A��Bװ���ڵ�һ���ֶ�����̼δ���������Ʒ�Ӧ���ͻ�ʹʵ����������ƫС�����ڿ����еĶ�����̼�����������Ʒ�Ӧ����û��װ��D�����Dzⶨ���ƫ�� ��4������̼���������ˮ�����ԣ���ƿ���ʵ���ʯ���Կ��������Ƴ����ʯ��ˮ�� �ʴ�Ϊ����1��CaCO3+2HCl=CaCl2+H2O+CO2������2�����������̼���壬CO2+2NaOH═Na2CO3+H2O��������̼����3��A��Bװ���ڵ�һ���ֶ�����̼δ���������Ʒ�Ӧ��ƫ��4��̼���������ˮ�� |