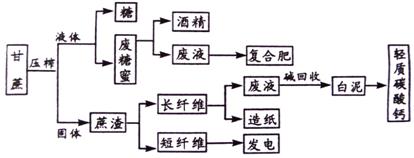
��Ŀ����
ͨ���������Ե��ǣ�����Ҫ�ɷ������ǣ���ѧʽΪC12H22O11�������ǵ��ܽ��Ϊ179g��100��ʱ���ǵ��ܽ��Ϊ487g��ij��ѧ��ȤС��ͬѧ��ʵ�����ø�����ȡ���ǣ����Ƚ����ʸ���Ū��С�飬���飬�ŵ�ˮ�н��裬�õ����ϣ�����֭�������Ĺ�Һ������
��1������ȥ������е��������ɲ��õ�ʵ�鷽����
��2����ȥ��������֭���ػ�ɫ�������֭������ɫ�������ɲ��õķ�����
��3�����Ӹ�Ũ�������DZ�����Һ�еõ����Ǿ���ɲ��õķ�����
��4����������������Ϊ10%������ˮ100g������ȡˮ
��1������ȥ������е��������ɲ��õ�ʵ�鷽����
����
����
����2����ȥ��������֭���ػ�ɫ�������֭������ɫ�������ɲ��õķ�����
�û���̿��ɫ
�û���̿��ɫ
����3�����Ӹ�Ũ�������DZ�����Һ�еõ����Ǿ���ɲ��õķ�����
�����ᾧ
�����ᾧ
����4����������������Ϊ10%������ˮ100g������ȡˮ
90
90
mL����������1�������ǰѲ�������Һ��Ĺ�����Һ������һ�ַ�����
��2������̿������һЩɫ�ء���ζ�Ͷ��������кܺõ������ԣ�
��3���ᾧ������Ϊ���֣������ᾧ�ͽ��½ᾧ�����½ᾧ�������ܽ�����¶�Ӱ��ϴ�����ʣ�
��4�����ʵ�����=��Һ�����������ʵ������������ܼ�������=��Һ������-���ʵ�������
��2������̿������һЩɫ�ء���ζ�Ͷ��������кܺõ������ԣ�
��3���ᾧ������Ϊ���֣������ᾧ�ͽ��½ᾧ�����½ᾧ�������ܽ�����¶�Ӱ��ϴ�����ʣ�
��4�����ʵ�����=��Һ�����������ʵ������������ܼ�������=��Һ������-���ʵ�������
����⣺��1�����ݹ��˵�ԭ��������ȥ������е��������ɲ��ù��˵�ʵ�鷽����
�ʴ�Ϊ������
��2��Ҫ����֭������ɫ�����������û���̿���������ã����û���̿������ɫ�ķ�����
�ʴ�Ϊ���û���̿��ɫ��
��3�����Ӹ�Ũ�������DZ�����Һ�еõ����Ǿ��壬���ǿ������������ᾧ�ķ�����
�ʴ�Ϊ�������ᾧ��
��4����������������Ϊ10%������ˮ100g�������ǵ�������100g��10%=10g����������ȡˮ������Ϊ90g������ˮ���ܶ���1g/cm3��������Ҫˮ�������90mL��
�ʴ�Ϊ��90��
�ʴ�Ϊ������
��2��Ҫ����֭������ɫ�����������û���̿���������ã����û���̿������ɫ�ķ�����
�ʴ�Ϊ���û���̿��ɫ��
��3�����Ӹ�Ũ�������DZ�����Һ�еõ����Ǿ��壬���ǿ������������ᾧ�ķ�����
�ʴ�Ϊ�������ᾧ��
��4����������������Ϊ10%������ˮ100g�������ǵ�������100g��10%=10g����������ȡˮ������Ϊ90g������ˮ���ܶ���1g/cm3��������Ҫˮ�������90mL��
�ʴ�Ϊ��90��
�����������Ͼ����龰�����˻����ķ��룬����̿�����ã����ʵ������������ɽ���Լ�ѧ����֪ʶ��������Ϻÿ���ѧ����֪ʶ�����պ�Ӧ�ã�

��ϰ��ϵ�д�
 ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�
�����Ŀ