��Ŀ����
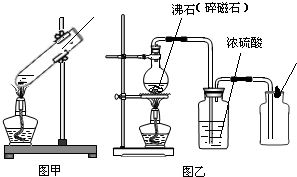
26��ij��ѧ��ȤС��ͬѧ��ʵ�������ռ���һЩ����ΪFeSO4��CuSO4�ķ�Һ������л��ս���ͭ������������Һ����������·�����
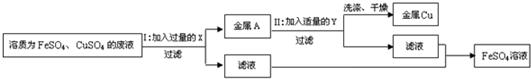
��1����������йط�Ӧ�Ļ�ѧ����ʽ��
��2��������������Լ�Y������Ϊ
��3�������͢��ж��й��˲���������Ҫ�IJ���������
��4��������о������ˡ�ϴ�ӡ�������Եõ������Ľ���ͭ��ϴ��ʱ�������ͭ�Ƿ���ϴ���ķ�����

��1����������йط�Ӧ�Ļ�ѧ����ʽ��
Fe+CuSO4�TCu+FeSO4
����2��������������Լ�Y������Ϊ
ϡ����
����3�������͢��ж��й��˲���������Ҫ�IJ���������
������
��©��
���ձ�
����4��������о������ˡ�ϴ�ӡ�������Եõ������Ľ���ͭ��ϴ��ʱ�������ͭ�Ƿ���ϴ���ķ�����
ȡ���ϴ��Һ�������μ������Ȼ�����Һ�������ᱵ��Һ������������Һ������������������֤������ͭ��ϴ��
��������������Ƶ���ĿҪ��ʵ��Ҫ�ﵽ��Ŀ��������˼��������Ŀ���ǻ��ͭ���ʺ�����������������Ҫ��ͭ����ת��Ϊͭ���ʣ�����Һ����Ҫ�����������������������µ����ʣ����Լ�����ܹ���ͭ����ת��Ϊͭ���ʵ�ֻ���ǵ�������
����⣺������Ҫ��ͭ����ת��Ϊͭ���ʣ�����Һ������Ϊ���������������������µ����ʣ����Կ��Լ�������ͭ����ת��Ϊͭ���ʣ�
Ҫ�뽫ͭ���ӳ���ת�������Լ������Ӧ���ǹ����ģ����Ժ��ڵõ���ͭ�����лẬ��ʣ��������ʣ�������Ҫ�����Ὣ���ܽ��������Ŀ���ǵõ��������������Լ������Ϊ�����������ȣ�
�ʴ�Ϊ����1��Fe+CuSO4�TCu+FeSO4
��2��ϡ����
��3��©�� �ձ� �������������Ⱥ�˳��
��4��ȡ���ϴ��Һ�������μ������Ȼ�����Һ�������ᱵ��Һ������������Һ������������������֤������ͭ��ϴ��
Ҫ�뽫ͭ���ӳ���ת�������Լ������Ӧ���ǹ����ģ����Ժ��ڵõ���ͭ�����лẬ��ʣ��������ʣ�������Ҫ�����Ὣ���ܽ��������Ŀ���ǵõ��������������Լ������Ϊ�����������ȣ�
�ʴ�Ϊ����1��Fe+CuSO4�TCu+FeSO4
��2��ϡ����
��3��©�� �ձ� �������������Ⱥ�˳��
��4��ȡ���ϴ��Һ�������μ������Ȼ�����Һ�������ᱵ��Һ������������Һ������������������֤������ͭ��ϴ��
��������������Ҫ����Ϊ���ֿ�����������Լ������Ժ�һ������Ҫ��֮��ȥ��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
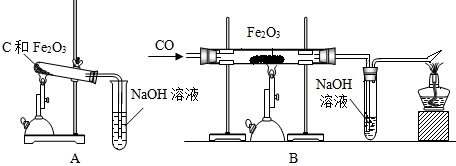
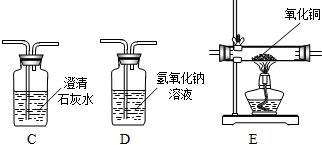
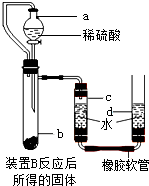
 ��
��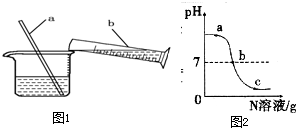 ��������ͭ������㷺ʹ�õ����ֽ���������������ϢϢ��أ�ͼ1��ϡ��Ũ����ʵ���ʾ��ͼ��
��������ͭ������㷺ʹ�õ����ֽ���������������ϢϢ��أ�ͼ1��ϡ��Ũ����ʵ���ʾ��ͼ�� ��֪M��N�ֱ���ϡ���ᡢ����������Һ�е�һ�֣�ij��ѧ��ȤС��ͬѧ��һ������M�в��ϵμ�N�����ⶨ������Һ��pH����ͼ��ʾ��
��֪M��N�ֱ���ϡ���ᡢ����������Һ�е�һ�֣�ij��ѧ��ȤС��ͬѧ��һ������M�в��ϵμ�N�����ⶨ������Һ��pH����ͼ��ʾ��