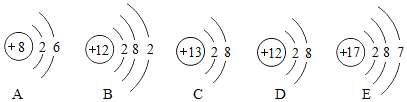
��Ŀ����
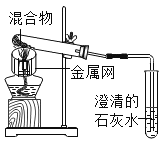
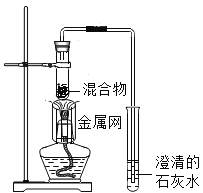
����Ŀ��Ϊ̽��̼��ԭ����ͭ�����ʵ����������ľ̿�ۺ�����ͭ�ĸ�������1-2.5g����ϵ��ʵ�顣
���������ϣ�������ͭ(CuO)Ϊ��ɫ���塣
��̼��ԭ����ͭ�õ���ͭ�п��ܺ���������������ͭ��������ͭΪ��ɫ���壬����ϡ���ᷴӦ��Cu2O+H2SO4=CuSO4+H2O+Cu
������ʵ�飩
ʵ��1����������1:11��ľ̿�ۺ�����ͭ�����1.2g������ʵ�顣
��� | 1-1 | 1-2 |
װ�� |
|
|
��Ӧ�����ʵĿ�ɫ��״̬ | ��ɫ��ĩ�л���������ɫ���� | ��ɫ�����н��������м�������ɫ���� |
ʵ��2��ȡһ�����Ļ�����1-2װ�ý���ʵ�顣
��� | ľ̿��������ͭ�������� | ��Ӧ�����ʵ���ɫ��״̬ | |
2-1 | 1��9 | ��ɫ�����н������� | ����������ɫ���� |
2-2 | 1��10 | ���к�������ɫ���� | |
2-3 | 1��11 | ���м�������ɫ���� | |
2-4 | 1��12 | ��ɫ���� | |
2-5 | 1��13 | ���н϶��ɫ���� | |
(1)ľ̼��ԭ����ͭ�Ļ�ѧ����ʽ��_____��
(2)ʵ��1-2�У�֤������CO2��������_____��
(3)ʵ��1��Ŀ����______��
(4)ʵ��2�Ľ�����______��
����˼�����ۣ�
(5)ʵ��2û�н���������Ϊ1:14��ʵ�飬������____��
(6)Ϊ����2-4�ĺ�ɫ�������Ƿ�Cu2O�������Լ���______��
���𰸡�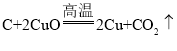 ����ʯ��ˮ����� ̽����ѵļ��ȷ�ʽ ���ȷ�ʽ��ͬ������£�ľ̿��������ͭ�����������Ϊ1:12 ľ̿��������ͭ��������Ϊ1:13ʱ�����н϶��ɫ���� ϡ����
����ʯ��ˮ����� ̽����ѵļ��ȷ�ʽ ���ȷ�ʽ��ͬ������£�ľ̿��������ͭ�����������Ϊ1:12 ľ̿��������ͭ��������Ϊ1:13ʱ�����н϶��ɫ���� ϡ����
��������
(1) ľ̼�ڸ���������ԭ����ͭ����ͭ�Ͷ�����̼����ѧ����ʽΪ��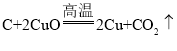 ��
��
(2) ��������̼ͨ�����ʯ��ˮ�У�![]() ����Ҫ֤������CO2�������dz���ʯ��ˮ����ǣ�
����Ҫ֤������CO2�������dz���ʯ��ˮ����ǣ�
(3)ʵ��1-1��ʵ��1-2���ȷ�ʽ��ͬ�����õ�������ɫ��״̬��ͬ�����ʵ��1��Ŀ����̽����ѵļ��ȷ�ʽ��
(4)����ʵ��2�У��ڼ��ȷ�ʽ��ͬ�������£����������ݿ�֪�����ȷ�ʽ��ͬ������£�ľ̿��������ͭ�����������Ϊ1:12����ʱ��ɫ���ʣ�
(5)ʵ��2û�н���������Ϊ1:14��ʵ�飬��������1:13ʱ�Ѿ��н϶��ɫ���ʣ�������ͭ�����������ӣ����и����ɫ����ʣ�ࣻ
(6)���� Cu2O+H2SO4=CuSO4+H2O+Cu����Ϊ����2-4�ĺ�ɫ�������Ƿ�Cu2O�������Լ���ϡ���ᣬ����ɫ���岿���ܽ⣬��Һ�����ɫ������������ͭ��

 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�