��Ŀ����
����Ŀ�����������еij���Ԫ�أ�ȱ��ʱ��ͨ��ʳ�ñ���ҩ����������������ij����ҩ��˵����IJ�����Ϣ��ͼ��ʾ��
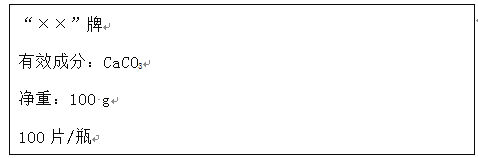
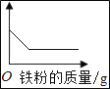
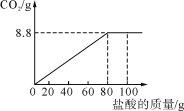
�ֽ�100 g����ֳ�5�ȷݣ���μӵ���40Ƭ��ҩ���Ƴɵķ�ĩ��(�����ɷֲ������ᷴӦ)���õ������������±���ͼ������ͼ��ʾ��������й���Ϣ�ش����⡣
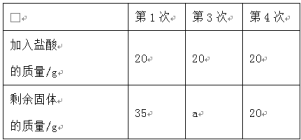
(1)����ȱ���׳��ֵļ�����_____����ʱ����ҽ��ָ���·��ñ���ҩ���⣬�ճ�������Ҫ������_____��ʳ�
(2)��Ʒ�Ʋ���ҩ����CaCO3������������_____��a����ֵΪ_____��
(3)������������ʵ���������_____��
���𰸡��������ɹ�ͷ��50%2518.25%
��������
(1)����ȱ���׳��ֹ������ɣ��ճ�������Ҫ������ϺƤ����ţ�̻��ͷ���Ⱥ��н϶�ĸ�Ԫ�ص�ʳ�
(2)��40Ƭ��ҩ���к�̼��Ƶ�����Ϊx�����������е���������Ϊy��
CaCO3+2HCl=CaCl2+CO2��+H2O
100 73 44
x y 8.8g
![]() x=20g��
x=20g��
![]() y=14.6g��
y=14.6g��
��Ʒ�Ʋ���ҩ����CaCO3�����������ǣ�![]() ��100%=50%��
��100%=50%��
�ɵ�1�κ͵�4�����ݿ�֪20gϡ���������5g̼��ƣ���ڶ��μ���20gϡ�����ʣ�����Ϊ30g�������μ���30gϡ�����ʣ�����Ϊ25g����a����ֵΪ25��
(3)�ɱ�����Ϣ��֪��80gϡ������40Ƭҩ���е�̼���ǡ����ȫ��Ӧ�����Ը����������ʵ���������Ϊ��![]() ��100%=18.25%��
��100%=18.25%��