��Ŀ����
����Ŀ����������Ħ����������̼��ƿ����ÿ�ʯ![]() ���Ʊ���ij��ѧ��ȤС�������
���Ʊ���ij��ѧ��ȤС�������![]() ��ת�����̣���ͼ��ʾ��
��ת�����̣���ͼ��ʾ��
���������̣�
��������ϣ�
![]() ��������̼����ͨ������������Һ�������·�Ӧ��
��������̼����ͨ������������Һ�������·�Ӧ��
![]() ��
��![]() ��
��
![]() ��̼���������ˮ�����ֽ⣺
��̼���������ˮ�����ֽ⣺![]() ��
��
![]() ����ʯ����ˮ��ַ�Ӧ��ɵõ������dz�ϸС����ʯ�ҽ���
����ʯ����ˮ��ַ�Ӧ��ɵõ������dz�ϸС����ʯ�ҽ���
���������ۣ�
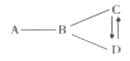
![]() С�����������̢١��ڡ��ܺͲ���
С�����������̢١��ڡ��ܺͲ���![]() ����ƣ���д����Ӧ�ٺܵ͢Ļ�ѧ����ʽ��
����ƣ���д����Ӧ�ٺܵ͢Ļ�ѧ����ʽ��
��________����________��
����![]() ���������________��ϴ�ӡ���ɵȹ��������У�ͨ����Ӧ�ܿɻ��ո���Ʒ_____��
���������________��ϴ�ӡ���ɵȹ��������У�ͨ����Ӧ�ܿɻ��ո���Ʒ_____��
![]() С����Ϊ���̢١��ڡ��ۺͲ���
С����Ϊ���̢١��ڡ��ۺͲ���![]() ��С�������Ÿ��ã������ǣ�________��
��С�������Ÿ��ã������ǣ�________��
![]() ������̼���ʱ��
������̼���ʱ��![]() Ϊ________��ѡ�����Һ������Һ�����������ǣ�________��
Ϊ________��ѡ�����Һ������Һ�����������ǣ�________��
![]() С����Ϊ�õ��IJ�Ʒ�п��ܺ�������
С����Ϊ�õ��IJ�Ʒ�п��ܺ�������![]() �����Բ���
�����Բ���![]() �������衢________���ˡ���ɵȹ�������߲��ʣ�
�������衢________���ˡ���ɵȹ�������߲��ʣ�
����Ʒ�����ⶨ��![]() �����IJⶨ��ȡ
�����IJⶨ��ȡ![]() ��Ʒ���гɷ�״��ͼ����ʵ�飮
��Ʒ���гɷ�״��ͼ����ʵ�飮
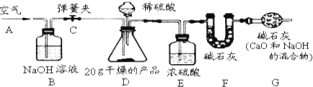
![]() ʵ�鲽�裺
ʵ�鲽�裺
�����Ӻ�װ�ã���������ԣ��ڴ��ɼ�![]() ����
����![]() ������ͨ��һ��ʱ�������
������ͨ��һ��ʱ�������
�۳���![]() ��������
��������![]() �������μ�ϡ������������ֱ��
�������μ�ϡ������������ֱ��![]() ��������ð����
����������
�ݴ��ɼ�![]() ���ٴλ���ͨһ��ʱ�����������
���ٴλ���ͨһ��ʱ�����������![]() ����������ǰ������������Ϊ
����������ǰ������������Ϊ![]() ��
��
![]() ����̽��
����̽��
�ٲ�Ʒ�гɷ۵�Ŀ��________��
��![]() װ�õ�������________��
װ�õ�������________��![]() װ�õ�������________��
װ�õ�������________��
����û��![]() װ�ã���ⶨ��
װ�ã���ⶨ��![]() ������������________���ƫ����ƫС���������䡱����
������������________���ƫ����ƫС���������䡱����
����![]() ����������ǰ������������Ϊ
����������ǰ������������Ϊ![]() ������ò�Ʒ��
������ò�Ʒ��![]() ����������Ϊ________
����������Ϊ________![]() ��
��
���ܽᷴ˼��
ijͬѧ�������ʵ�鷽���ⶨ�����и�Ԫ�ص�������������һ���������м������ϡ���ᣬ�ⶨ����![]() ���������ݴ˼��������и�Ԫ�ص�����������С��ͬѧ��Ϊ��ʹ�ų�ʵ�������Ͳ�����Ӱ�죬�����ⶨ�Ľ���Բ�һ��ȷ��������________��
���������ݴ˼��������и�Ԫ�ص�����������С��ͬѧ��Ϊ��ʹ�ų�ʵ�������Ͳ�����Ӱ�죬�����ⶨ�Ľ���Բ�һ��ȷ��������________��
���𰸡�CaCO3![]() CaO+CO2��Ca��OH��2+Na2CO3=CaCO3��+2NaOH����NaOH������̼�õ�������ã���Լԭ������Һ����ʯ��ˮ����������Ũ��̫С������Ч�ʺܵ�������Ӵ��������ַ�Ӧ����ȥ�����еĶ�����̼����ֹ�����еĶ�����̼����
CaO+CO2��Ca��OH��2+Na2CO3=CaCO3��+2NaOH����NaOH������̼�õ�������ã���Լԭ������Һ����ʯ��ˮ����������Ũ��̫С������Ч�ʺܵ�������Ӵ��������ַ�Ӧ����ȥ�����еĶ�����̼����ֹ�����еĶ�����̼����![]() װ�ã�ƫ��
װ�ã�ƫ��![]() ��������̼������ˮ
��������̼������ˮ
��������
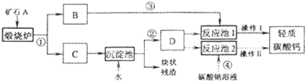
��1��̼����ֽܷ����������ƣ�����������ˮ��Ӧ�����������ƣ������������������̼��̼���Ʒ�Ӧ����̼��ƣ����ɵ�̼��Ʋ�����ˮ������ͨ�����˵ķ�������Һ�з����������2���������ʵ�ѭ�����÷�������3�������������Ƶ��ܽ��Է�������4������������Ϣ������̼����������̼������Լ�̼������ȷֽ�����ʷ�������6����������ͼ��ʵ��ԭ����Ҫ�ⶨ̼��Ƶ������������ɲⶨ���ɵĶ�����̼��������������Fװ�����ն�����̼��������֮ǰ���ȥ������̼�е�ˮ�ּ������еĶ�����̼�����Ҫ���ó�ȥ������̼�Ŀ��������ɵĶ�����̼ȫ���ŵ�Fװ���������ݶ�����̼���ܽ��Խ����
��1����̼��Ƹ��·ֽ����������ƺͶ�����̼����Ӧ�Ļ�ѧ����ʽΪCaCO3![]() CaO+CO2������������������̼���Ʒ������ֽⷴӦ����̼��ƺ��������ƣ����ɵ�̼��Ʋ�����ˮ����Ӧ�Ļ�ѧ����ʽΪCa��OH��2+Na2CO3=CaCO3��+2NaOH�������������ˡ�ϴ�ӡ���ɵȹ��������У�ͨ����Ӧ�ܿɻ��ո���ƷNaOH����2�����̢١��ڡ��۵Ģ��ǽ�̼��Ʒֽ����ɵĶ�����̼�������ã���Լ��ԭ������3��������̼���ʱ��DΪ����Һ�������ǣ�����ʯ��ˮ����������Ũ��̫С������Ч�ʺܵ�����4����������Ϣ������̼����ͨ������������Һ�������·�Ӧ��CO2+Ca��OH��2=CaCO3��+H2O��CaCO3+H2O+CO2=Ca��HCO3��2��̼���������ˮ�����ֽ⣺Ca��HCO3��2�TCaCO3��+H2O+CO2����֪��̼�����ת����̼��ƿ��Բ����ȵķ�������6��������Ӵ��������ַ�Ӧ���ɽ���Ʒ�гɷ�ĩ���ڸ�������ͼװ�÷�����Ҫͨ���ⶨ���ɵĶ�����̼����������̼��Ƶĺ��������轫���ɵĶ�����̼�ó�ȥ������̼�Ŀ���ȫ���ų������Bװ�õ������dz�ȥ�����еĶ�����̼�� Gװ�õ������Ƿ�ֹ�����еĶ�����̼����Fװ�ã�����û��Eװ�ã����ɵĶ�����̼�����к���ˮ�ֶ�ʹ������̼������������ⶨ��CaCO3������������ƫ��������F����������ǰ������������Ϊ8.7g�������ɵĶ�����̼������Ϊ8.7g��
CaO+CO2������������������̼���Ʒ������ֽⷴӦ����̼��ƺ��������ƣ����ɵ�̼��Ʋ�����ˮ����Ӧ�Ļ�ѧ����ʽΪCa��OH��2+Na2CO3=CaCO3��+2NaOH�������������ˡ�ϴ�ӡ���ɵȹ��������У�ͨ����Ӧ�ܿɻ��ո���ƷNaOH����2�����̢١��ڡ��۵Ģ��ǽ�̼��Ʒֽ����ɵĶ�����̼�������ã���Լ��ԭ������3��������̼���ʱ��DΪ����Һ�������ǣ�����ʯ��ˮ����������Ũ��̫С������Ч�ʺܵ�����4����������Ϣ������̼����ͨ������������Һ�������·�Ӧ��CO2+Ca��OH��2=CaCO3��+H2O��CaCO3+H2O+CO2=Ca��HCO3��2��̼���������ˮ�����ֽ⣺Ca��HCO3��2�TCaCO3��+H2O+CO2����֪��̼�����ת����̼��ƿ��Բ����ȵķ�������6��������Ӵ��������ַ�Ӧ���ɽ���Ʒ�гɷ�ĩ���ڸ�������ͼװ�÷�����Ҫͨ���ⶨ���ɵĶ�����̼����������̼��Ƶĺ��������轫���ɵĶ�����̼�ó�ȥ������̼�Ŀ���ȫ���ų������Bװ�õ������dz�ȥ�����еĶ�����̼�� Gװ�õ������Ƿ�ֹ�����еĶ�����̼����Fװ�ã�����û��Eװ�ã����ɵĶ�����̼�����к���ˮ�ֶ�ʹ������̼������������ⶨ��CaCO3������������ƫ��������F����������ǰ������������Ϊ8.7g�������ɵĶ�����̼������Ϊ8.7g��
��̼��Ƶ�����Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 8.7g![]()
x=19.77g
��̼��Ƶ���������Ϊ��![]() ��100%=98.86%
��100%=98.86%
���ܽᷴ˼�������ǣ�������̼������ˮ������ʹ��õĶ�����̼���ƫС��

����Ŀ���ڡ�����ȼ�ա���̽��ʵ���У���������Ļش����ڡ�ʵ�鷽����ơ����ǣ� ��
ѡ�� | ���� | ������Ļش� |
| ��������ʱ�����İ�����ʲô�� | ������ʯ������ |
| �ø�����ձ����ڻ����Ϸ����۲쵽ʲô�� | �ձ��ڱ���ˮ�� |
| ����ȼ�պ�IJ�����ʲô�� | ȼ�պ����ɶ�����̼ |
| ��������¶��IJ���ߣ� | �����ƽ���ڻ����� |
A. ![]() B.
B. ![]() C.
C. ![]() D.
D. ![]()