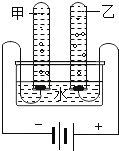
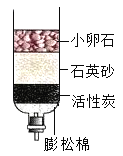
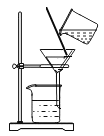
��Ŀ����
����Ŀ����ͼ��ʾΪʵ���� �г��������Ʊ�������ռ�������ʵ��IJ�������װ�ã���װʵ��װ��ʱ�����ظ�ѡ��ijѧУ������ѧʵ��̽��С���ͬѧ����������ɸ��Ե�̽��ʵ�飬�Ը�����ĿҪ�ش��������⣺
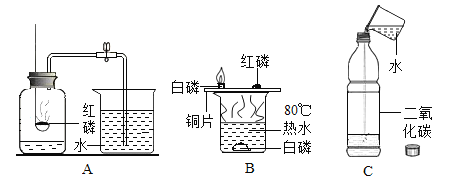
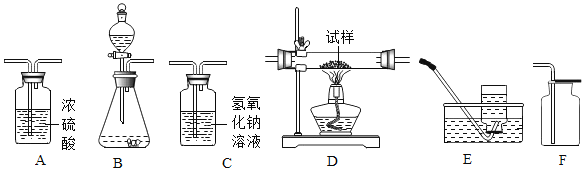
��1����һ���ͬѧ��ʯ��ʯ��ϡ����Ϊԭ�ϣ���ʵ�����Ʊ����ռ����﴿���Ķ�����̼���塣 ����ʾ���ӷ���������HCI������ñ���̼��������Һ���գ�
����ѡ����������˳��Ϊ_____����д����װ�������ĸ����
��д��ʵ������ȡ������̼�ķ�Ӧ����ʽ_____��
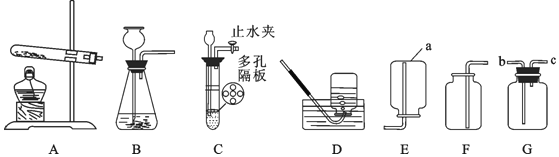
��2���ڶ����ͬѧ�Թ���������ҺΪԭ�ϣ�MnO2 Ϊ�������Ʊ�����������ij�����ϵ����Ԫ�ؽ��з���̽����������ʾ�����Ϻ�C��H��O����Ԫ�أ�����Ƶ�װ��˳��Ϊ��A��E��B��D����ʯ�Ҹ���ܡ���
��װ��D�е�����ʵ��������_____��
��װ��E�IJ������з����������м����Ϊwg�������������ȼ�պ������B����������mg��װ��D����ng���������������̼Ԫ�ص�����Ϊ_____���÷�����ʽ��ʾ������������Ƶ�װ��˳�������ĸ�������������Ԫ��Ԫ����̼Ԫ�ص���������ʵ��ֵ��Ƚ�_____ ���ƫС����ƫ�� ������һ�¡�֮һ����
���𰸡�ACBF ![]() ����ʯ��ˮ�����
����ʯ��ˮ����� ![]() g ƫ��
g ƫ��
��������
��1���ٸ��������Ϣ��֪��ϡ�����лӷ������������Ȼ���������ñ���̼��������Һ���գ�ˮ��������Ũ���������գ���һ���ͬѧ��ʯ��ʯ��ϡ����Ϊԭ�ϣ���ʵ�����Ʊ����ռ����﴿���Ķ�����̼���壬��ѡ����������˳��Ϊ��ACBF��
��ʵ������ʯ��ʯ��ϡ����Ϊԭ�ϣ���ȡ������̼����ѧ����ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2����
��2���ٶ�����̼�ͳ���ʯ��ˮ��Ӧ�������dz���ʯ��ˮ����ǣ�
�ڶ�����̼��ʹ�����ʯ��ˮ����ǣ�Dװ�����ӵ������������ɵĶ�����̼��������������̼��̼Ԫ�ص�����=ng��![]() ��100%=
��100%=![]() g���������ɵ������к�������ˮ������ʹBװ�����ص�����ƫ��ˮ���������ӣ���˲�����ĸ�������������Ԫ�ص�������ʵ��ֵ�ȽϽ�ƫ������ĸ�������������Ԫ��Ԫ����̼Ԫ�ص���������ʵ��ֵ��Ƚ�ƫ��
g���������ɵ������к�������ˮ������ʹBװ�����ص�����ƫ��ˮ���������ӣ���˲�����ĸ�������������Ԫ�ص�������ʵ��ֵ�ȽϽ�ƫ������ĸ�������������Ԫ��Ԫ����̼Ԫ�ص���������ʵ��ֵ��Ƚ�ƫ��
�ʴ�Ϊ��
��1����ACBF����CaCO3+2HCl=CaCl2+H2O+CO2����
��2���ٳ����ʯ��ˮ����ǣ���![]() g��ƫ��
g��ƫ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��ʵ��̽������ѧ����������Ӧ�߱���ѧ����������ѧ��ȤС���ͬѧ��ʵ������ȡ������̼����̽��������һ����롣
���������ϣ���1��ϡ�����������ʯ��Ӧ�����ɵ����������ˮ��
��2��ϡ���������ԣ���ʹ��ɫʯ����Һ��졣
��ѡ��ҩƷ�����±�����ʵ�飬ȡ�������Ĵ���ʯ�����������У����ʲ����ᷴӦ��������������̼�����ʱ��仯��������ͼ��ʾ��
ʵ���� | ҩƷ |
�� | ��״����ʯ��10%H2SO4��Һ |
�� | ��״����ʯ��7%HCl��Һ |
�� | ����ʯ��ĩ��7%HCl��Һ |
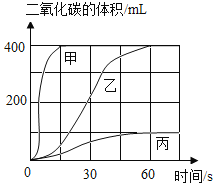
ͼ�б���Ӧʵ��_________(ѡ���� ������ ������ ��)��ȷ�����Ҷ�Ӧ��ҩƷ�Ʊ�������̼����Ӧ�Ļ�ѧ����ʽ��___________����ѡ���Ӧ��ҩƷ��������______________��
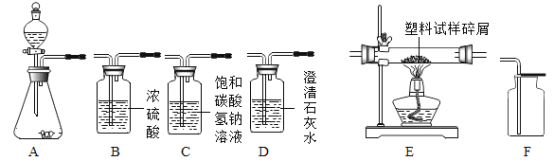
��ѡ��������С��ͬѧ��ͼ 2 ��ʾ��������װһ�ɿ��Ʒ�Ӧ���ʵ���ȡװ�ã�����Ϊ����ѡ���������__________������ţ�
���ռ����壩����ͼװ���ռ�������̼��������_____________��ѡ����X������Y�������ų���
���������壩�������������Ϊ������̼�ķ�����_________________��
����չ���£�ʵ�������С��ͬѧ��װ���еķ�Һ��������Ȥ����ͬѧ��Ϊ����ϡ������ʣ�࣬��֤���ò�������ȷ�ģ������Һ�м���______________�������_________������
����Ŀ����ѧ��ȤС���ͬѧ������ij��ʯ�Ʊ�������þ����֪�ÿ�ʯ������þ��������������ͭ�Ͷ���������ɣ��Ʊ���������ͼ��ʾ����֪���������費����ˮҲ����ϡ���ᷴӦ����

��ش��������⣺
��1����Һ A �е������ӳ��� Mg2+��Fe3+��Cu2+�⣬������_____��д����ʯ�е�����һ�ֽ�����������ϡ���ᷴӦ�Ļ�ѧ����ʽ��_____��ֻдһ������
��2������Һ A �м�����ʯ�ҵ�����Һ�� pH������ʹ��Һ�еĽ�����������ת��Ϊ��������ʵ�������£�ʹ���������ӳ�������� pH ���ݼ��±���Ϊ��֤��Ʒ���ȡ����ٲ�Ʒ��ʧ�������ڲ�����������Һ B �� pH ��ȡֵ��ΧΪ_____��
�������� | Fe��OH��3 | Cu��OH��2 | Mg��OH��2 |
��ʼ����ʱ�� pH | 1.5 | 4.2 | 8.6 |
��ȫ����ʱ�� pH | 3.2 | 6.7 | 11.1 |
��3��д����Һ B �м�����ʯ�ҷ����Ļ�ѧ��Ӧ����ʽ��_____��