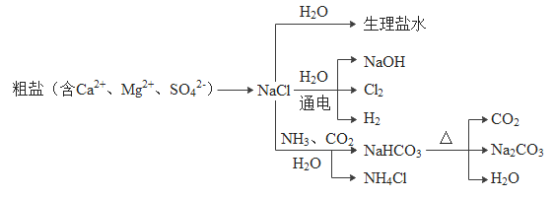
��Ŀ����
����Ŀ����ѧ�����������ߣ��ճ��������̺���������ѧ֪ʶ���밴��Ҫ����գ�
��1��Ư�����������Ҫ���ж������裬��д����������Ļ�ѧʽ��_____��
��2���������������Ҫ���뷢�ͷ�С�մ���д��С�մ�Ļ�ѧʽ��_____��
��3�����������ܵ���ʯ�ͺ���Ȼ�������й���ʯ������_____����������������
��4����ˮ�õ�ˮ�����ֱ����õ�����Ӧ����_____����ȹ��ԡ��������ԡ�����
��5����Ȼ���Ѿ��߽����ǵ������У���д������ȼ�յĻ�ѧ����ʽ��_____��
��6�����Ͻ�ƿ��ڿ����о���ǿ����ʴ�Ե�ԭ��_____���û�ѧ����ʽ��ʾ����
���𰸡�SiO2 NaHCO3 ����� �ȹ��� CH4��2O2![]() CO2��2H2O 4Al��3O2=2Al2O3
CO2��2H2O 4Al��3O2=2Al2O3
��������
��1����Ԫ�ػ��ϼ�-2�ۣ���Ԫ��+4�ۣ����ݻ��ϼ۴�����Ϊ�㣬��������Ļ�ѧʽΪSiO2�����SiO2��
��2��С�մ�ѧʽΪNaHCO3�����NaHCO3��
��3��ʯ�����ɶ��ֳɷ���ɵĻ����������
��4����ˮ�õ�ˮ�����ֱ������Ȳ����ۻ��������ȹ������ϣ�����ȹ̡�
��5������ȼ�����ɶ�����̼��ˮ����ѧ����ʽΪCH4��2O2![]() CO2��2H2O�����CH4��2O2
CO2��2H2O�����CH4��2O2![]() CO2��2H2O��
CO2��2H2O��
��6���������������γ�һ����������Ĥ��ѧ����ʽΪ4Al��3O2=2Al2O3����ֹ���Ľ�һ����ʴ��ʹ�����п���ʴ�ԣ����4Al��3O2=2Al2O3��

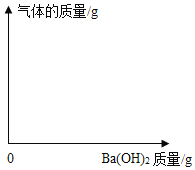
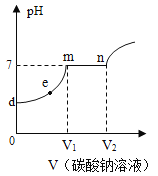
����Ŀ��ũҵ�ĸ߲������벻�����ʵĺ���ʩ�á���ͼ��ǩΪijƷ�ƻ��ʵ��̱꣬Ϊ�ⶨ�û����У�NH4��2SO4�ĺ�����С���ȡ5.0g�û��ʷ����ձ��У���ˮ��Ҫ�ɷ֣���NH4��2SO4��ȫ�ܽ⣬�ٽ�200gBa��OH��2��Һ��4�μ������У���������ˮ�����μӷ�Ӧ����������������ʾ��

���� | 1 | 2 | 3 | 4 |
����Ba��OH��2��Һ����/g | 50 | 50 | 50 | 50 |
���ɳ�������/g | 2.33 | 4.66 | m | 6.99 |
��֪����NH4��2SO4+Ba��OH��2=BaSO4��+2NH3��+2H2O
��1������m��ֵΪ_____��
��2������Ba��OH��2��Һ�����ʵ������������жϸû����Ƿ�ϸ�д��������̣���
��3������5.0g�û��ʹ����ĩ������Ba��OH��2�����ĩ�����ĥ��������ͼ�л�����������������Ӧ�ı仯ͼ������ע��Ҫ����ֵ��