��Ŀ����
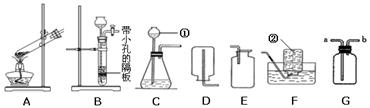
(8��)��ѧ��һ����ʵ��Ϊ������ѧ�ƣ���ѧ��ȡ�õķ�˶�ɹ�������ʵ�����Ҫ���÷ֲ����ġ��������ʵ��װ��ͼ�ش����⣺
(1)д��ָ�����������ƣ��� ���� ��
(2)д��ʵ�������ø��������ȡ�����Ļ�ѧ����ʽ��
��
(3)ʵ�����ռ�����ʱ����ѡ�õ��ռ�װ���� (����ĸ����)��
(4)ʵ������ȡ������̼ʱ����ѡ�õķ���װ���� (����ĸ����)��д����ѧ��Ӧ����ʽ ��
��5���ռ�������̼ʱ����ѡ��װ��D�������� ��Ҳ����ѡ��װ��E�������� ��

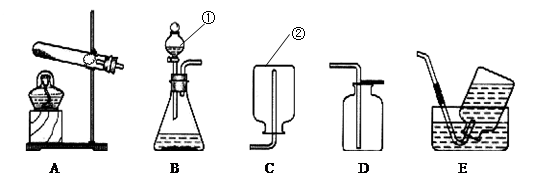
(1)д��ָ�����������ƣ��� ���� ��
(2)д��ʵ�������ø��������ȡ�����Ļ�ѧ����ʽ��
��
(3)ʵ�����ռ�����ʱ����ѡ�õ��ռ�װ���� (����ĸ����)��
(4)ʵ������ȡ������̼ʱ����ѡ�õķ���װ���� (����ĸ����)��д����ѧ��Ӧ����ʽ ��
��5���ռ�������̼ʱ����ѡ��װ��D�������� ��Ҳ����ѡ��װ��E�������� ��
(1) ��Һ©�� ����ƿ
(2) A 2KMnO4��K2MnO4 + MnO2 + O2��
(3) DE
��4�� B CaCO3 + 2HCl ="=" CaCl2 + CO2��+ H2O
��5��������̼�ܶȱȿ������Ҳ���������Ӧ��
��ΪCO2�ܽ������ޣ��ӵ��ܿڲ�����������ˮ���ܽϿ측����ݳ���
(2) A 2KMnO4��K2MnO4 + MnO2 + O2��
(3) DE
��4�� B CaCO3 + 2HCl ="=" CaCl2 + CO2��+ H2O
��5��������̼�ܶȱȿ������Ҳ���������Ӧ��
��ΪCO2�ܽ������ޣ��ӵ��ܿڲ�����������ˮ���ܽϿ측����ݳ���
������������Т٢����������Ʒֱ�Ϊ��Һ©���ͼ���ƿ��ʵ�������ø��������ȡ�������Ǽ��ȹ��������������ͣ�����װ��ӦѡA����ѧ����ʽΪ��2KMnO4��K2MnO4 + MnO2 + O2���������ܶȱȿ�����������ˮ�����ռ�װ�ÿ�ѡ DE���������ſ���������ˮ���ռ�������ʵ������ȡ������̼���ǹ�����Һ�巴Ӧ����Ҫ���ȵ����ͣ���ѡ�õķ���װ����B����ѧ��Ӧ����ʽΪ��CaCO3 + 2HCl ="=" CaCl2 + CO2��+ H2O ���������̼�ܶȱȿ������Ҳ���������Ӧ�����ռ�������̼ʱ����ѡ��װ��D������ΪCO2��ˮ���ܽ������ޣ��ӵ��ܿڲ�����������ˮ���ܽϿ측����ݳ��������ռ�������̼Ҳ��ѡ��Eװ�á�
������ʵ������ȡ�����Ͷ�����̼����Ҫ�Ŀ��㣬������֪ʶ�����һ����Ƚ��ż�������⣬��������ѧϰ��

��ϰ��ϵ�д�
�����Ŀ
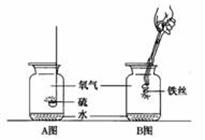
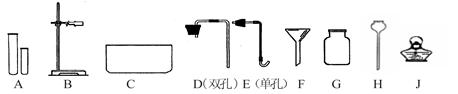
 ��ʵ���ҳ���FeS������ϡH2SO4��Ӧ��ȡH2S���壺FeS+H2SO4 FeSO4 + H2S�����ݴ˿�֪����ȡH2S����ķ���װ����____����ͬ�����2������3���������ռ�H2S����ķ�����_____�����2������3���������ƣ��ռ�H2S����ʱ��Ӧ�ر��ע��������_________ ��
��ʵ���ҳ���FeS������ϡH2SO4��Ӧ��ȡH2S���壺FeS+H2SO4 FeSO4 + H2S�����ݴ˿�֪����ȡH2S����ķ���װ����____����ͬ�����2������3���������ռ�H2S����ķ�����_____�����2������3���������ƣ��ռ�H2S����ʱ��Ӧ�ر��ע��������_________ ��