��Ŀ����
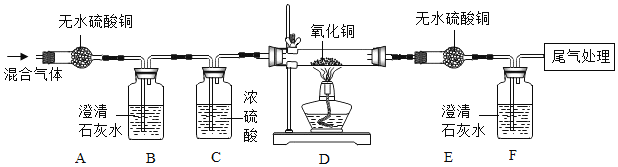
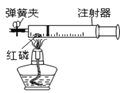
����Ŀ����������ʦ����ʵ��һ����װ�ý��з����˶�̽��ʵ��ʱ��ͬѧ���ŵ���һ�����ŵĴ̼�����ζ���κ�ѧ��ȤС���ԭʵ��װ�ý����˸Ľ�����ʵ��װ�������Թ��н��У���ͼ�С�ʵ������͡�ʵ��������ʾ��
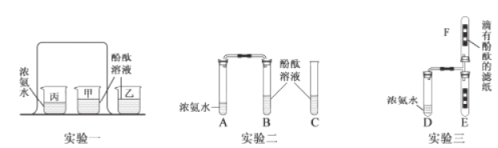
��1������ʵ��һ������ʱ��������������ձ���_________����ס������ҡ���������������˵��_____����ţ���
�ٷ��Ӽ��м�϶ �ڷ����ڲ����˶� �۷�������ԭ�ӹ��ɵ� ���¶�Խ�߷����˶�Խ��ݷ��Ӻ�С���ۿ����� ���ӹ��ɲ�ͬ����ѧ���ʲ�ͬ
��2����װ�øĽ��ɡ�ʵ����������ŵ���_________������ţ���
A�����ٿ�����Ⱦ B��ʵ�����������
C��ʵ����۸�ȷ D����ԼҩƷ
��3����ʵ��������װ�ñȡ�ʵ��һ���͡�ʵ�������װ������һ���֣��ò���װ���ڡ�ʵ��һ���͡�ʵ������е�������_________ ����ѯ���Ϸ��֣���Ũ��ˮ�ܹ��ͷų�������NH3������������ӵ���Է�����������29���������ܶȴ��ڿ�������֮��С�ڿ�����ʵ������У�_________�E����F�����Թ��е��з�̪����ֽ������ȫ����졣
���𰸡��� �ڢ� AD �Ա����� F
��������
��1������ʵ��һ������ʱ��������������ձ��Ǽ���������Ϊ���ձ��İ������Dz����˶��������˶������ձ���ʱ���ܺ����е�ˮ��Ӧ���ɰ�ˮ���Ӷ�ʹ��̪��Һ�����������˵�������ڲ����˶������Ӻ�С���ۿ���������ѡ�ڢ���
��2���ԱȸĽ�ǰ��ʵ�����Ľ���ʵ����ŵ����ܾ�����ֹ�����ݳ�����װ�øij���ʵ����������ŵ��Ǽ��ٿ�����Ⱦ����ԼҩƷ����ѡAD��
��3����ʵ��������װ�ñ���ʵ��һ������ʵ�������װ������һ���֣��ò���װ������ʵ��һ������ʵ������е������ǶԱ���������ѯ���Ϸ��֣���Ũ��ˮ�ܹ��ͷų�������NH3������������ӵ���Է�����������29���������ܶȴ��ڿ�������֮��С�ڿ�������������Է�������=14+1��3=17��29���ʰ������ܶ�С�ڿ�����ʵ������У�F�Թ��е��з�̪����ֽ������ȫ����졣

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ������ʦ��ָ���£�ͬѧ�ǽ�������Ȥ�Ļ�ѧʵ��̽����
(һ)�ⶨ��������������
��ͼ��ʾ����С��ͬѧ�ú����ڿ�����ȼ�յIJⶨ�����������ǣ�
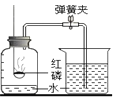
��1����������ƿ�ݻ�����Ϊ��ȷݣ������ñ�ǣ�
��2������ȼȼ�ճ��ڵĺ��ף����뼯��ƿ�в�������������
��3����������Ϩ����ȴ���ɼУ�����ˮ�����뼯��ƿ�У����뼯��ƿ��ˮ�����ԼΪ����ƿ���ݻ���1/5����ش��������⣺
�ٵ�2����ѧ���ű���ʽ_______________��
��ʵ����ϣ������뼯��ƿ��ˮ������������ݻ���1/5����ԭ���������_____��
A.����ƿ�ײ�ˮռ��һ������� B.������
C.û����ȫ��ȴ�ʹ���ֹˮ�в����� D.©��
��С��ͬѧ��ʵ����з�˼������˸Ľ�����(��ͼ��ʾ)��С������ʽ��ʼʵ��ǰ���н����ɼУ���ע����������20mL�̶ȴ�����15mL����Ȼ���ɿ��������۲쵽����������20mL�̶ȴ����ò�������ҪĿ����_______________������Ϊ�Ľ�����ŵ���________________ ��
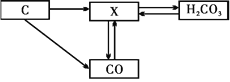
(��)С���Ķ��������ϵ�֪��˫��ˮ�ֽ�����ö�������(MnO2)����������ͭ(CuO)��������������������������ͭ������̽����Ȥ��
(�������)����ͭ�Ƿ�Ҳ��������طֽ�Ĵ��������Ƿ�ȶ������̴�Ч�����ã�
(���ʵ��)С�������ɵ����������Ϊ�����������������ʵ��(��������Ӱ��ʵ������ؾ�����)
ʵ����� | ��������� | ������������ | �������� |
�� | 1.2g | / | |
�� | 1.2g | CuO 0.5g | |
�� | 1.2g | MnO2 0.5g |
����ʵ��Ӧ��������������������_______ ��
����ʵ��ڱ�ʵ��ٵ�������������________(������������С��)��˵������ͭ�ܼӿ�����صķֽ⣮
�۽�ʵ��ڷ�Ӧʣ��Ĺ���ȡ����ϴ�ӡ�����ٴξ�ȷ�����õ�0.5g��ɫ��ĩ����������Ŀ����______��С��ͬѧ��Ϊ����ͭ�϶�������طֽ�Ĵ�������С��ͬѧ�Դ���������飬С��ͬѧ���������ʵ�飺���ڶ��ξ�ȷ�����õ���0.5g��ɫ��ĩ��1.2g����ػ�Ϸ����Թ��У����ȣ����������ľ��������ľ���ܿ츴ȼ��С��ͬѧ������Ŀ����__________��
(Ԥ�ڽ���)����ͭҲ��������صĴ�����
(�������)����ΪС�����ʵ��ۺ�ʵ��ڶԱȵ�Ŀ����____��д��ʵ��ڷ�Ӧ�ı���ʽ_____
(��)��Ȥ�ĸĽ�ʵ��
������ͼװ�ý�������ȼ������ʵ�飺������(�ܲ��Ϲ��������װ��)���ϵ������;�֧�Թ��й��������ͬʱ�þƾ��Ƹ���ۼ�����ȼ�գ���ȥ�ƾ��ƣ��ɹ۲쵽���ĵ���ɫ���棻Ȼ��ֹͣ������������þƾ��Ƹ�������ؼ��ȣ�ʹ���ڴ����м���ȼ�գ��۲쵽________���森

д��ʵ�������ȼ�յĻ�ѧ��Ӧ����ʽ��_____����ʵ��װ�õ���Ҫ�ŵ����������к�����Ի�����Ⱦ��ǰ����������_____ �����ƶ��ձ��е�����������Һ��������______ ��