��Ŀ����
����Ŀ����һ�ձ���ʢ��Na2CO3��NaCl��ɵĹ������ﹲ18.9g������112.5 gˮʹ����ȫ�ܽ����Ƴ���Һ���������μ�������������Ϊ10%��ϡ���ᣬ�ų��������������������ϡ�����������ϵ������ͼ��ʾ,���������ش����⣺
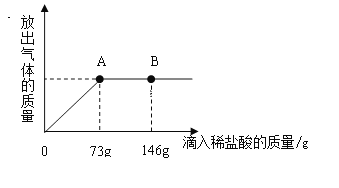
��1�����μ�ϡ������ͼ��B��ʱ���ձ�����Һ��������ǣ�д��ѧʽ��________��
��2�����μ���73gϡ����ʱ����A��ʱ�����ձ���Ϊ��������Һ����ͨ�����������ʱ��Һ��������������________��
���𰸡�NaCl��HCl10%
��������
(1)̼���������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����Ӧ����Һ�е��������Ȼ��ƣ���B�������������Һ�е����ʳ��Ȼ����⣬����ʣ����Ȼ��⣬������Һ�е��������Ȼ��ƺ��Ȼ����������ʣ�����NaCl��HCl��
(2)�⣺73��10%��ϡ���������ʵ�����Ϊ��73����10%=7.3��
��ԭ�������̼���Ƶ�����Ϊx����Ӧ�������Ȼ��Ƶ�����Ϊy�����ɶ�����̼������Ϊz
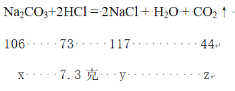
![]() =
= ![]() =
= ![]() =
= ![]()
��� x=10.6�� y=11.7�� z=4.4��
A����Һ��������������Ϊ
![]() ��100%=10%
��100%=10%
��������������Ϊ10%��

 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д� ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д� ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д�����Ŀ���ϳɰ��������ѧ�����ϵ�һ���ش�ͻ�ƣ�����ᷢչ��Ƽ����������˾��ף��ϳɰ����յ���Ҫ������ͼ1��
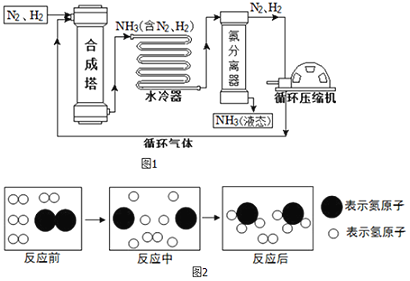
��1���ϳ����еķ�Ӧ�����ڸ��¡���ѹ�����������½��У��÷�Ӧ��ѧ����ʽ�ǣ�_______������_______���������Ӧ���ͣ���Ӧ��
��2�����������дӺϳ������������������_______�����������������������������
��3�������������п��ظ�ʹ�õ�������________���ѧʽ����
��4�����ݱ��е����ݻش����⣮
���� | H2 | N2 | O2 | NH3 |
�е�/����1.01��105Pa�� | -252 | -195.8 | -183 | -33.35 |
��1.01��105Paʱ������NH3��N2��H2���룬��ý��¶ȿ�����_______֮�䣮
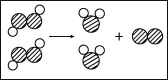
��5��ͼ2�Ǻϳ����з�����Ӧ��������ʾ��ͼ����ͼ��֪���ַ�Ӧ��N2��H2�ķ��Ӹ�����Ϊ_______��
�÷�Ӧ�е���С������_______����д��ѧ���ţ���