��Ŀ����
����Ŀ�������������������ܾ���ȼ�ա�ij��ѧ̽��С��ԡ���˿��������ȼ��Ϊʲô��������䡱����Ȥ�����ǽ���������̽����
��������⣩��˿��������ȼ�ջ������������Щ�����й��أ�
����������裩����һ������������ķе��йأ��������������������Ũ���йأ������������������к�̼���йء�
���������ϣ�þ�ķе�Ϊ1107�棻���ķе�Ϊ2705�档
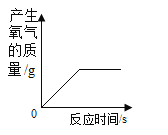
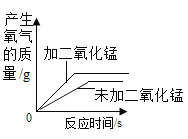
����Ʋ�ʵ�飩��̽��С���ý�þ˿����ϸ��˿�����ֲ�ͬ��̼����ϸ��˿��þ˿����˿ֱ����Ϊ0.4mm���ֱ���������������ȼ�ա���¼ȼ��ʱ���������±���
ʵ����� | �������ȣ��������� | þ˿ | ��˿�к�̼�� | ȼ��ʱ������ |
�� | �����У�21%�� | ����ȼ�գ�����ҫ�۰⣬���� | 0%�������� | ��ȼ�� |
�� | �����У�100%�� | ������ȼ�գ�����ҫ�۰⣬���� | 0%�������� | ����ȼ�գ����� |
�� | �����У�100%�� | ������ȼ�գ�����ҫ�۰⣬���� | 0.05% | ����ȼ�գ����ٻ��� |
�� | �����У�100%�� | ������ȼ�գ�����ҫ�۰⣬���� | 0.6% | ����ȼ�գ��������� |
���ռ�֤�ݣ���1����Ҫ�Ƚ�ϸ��˿ȼ�յľ��ҳ̶��������Ĵ����йأ�Ӧѡ���ʵ���������_____����ʵ��ۺܶ͢Աȿ�֪���������������������ϸ��˿�к�̼���Ĺ�ϵ��_____��
����������ۣ���2��ͨ��̽�����˽�ϸ��˿�ڴ���������ȼ��ʱ����������ԭ����д��ϸ��˿��������ȼ�����ɺ�ɫ����������������Ļ�ѧ����ʽΪ��_____��
��3��ϸ��˿�к��в�ͬ�ĺ�̼����̼Ԫ����ȼ�չ��������ɶ�����̼���壬����Ӧ���ɵ��ۻ����������������˳�ȥ���Ӷ������˻������������Ϊ��֤ʵ��ɹ���ѡ�õ���˿�к�̼��Ҫ���Ƶķ�ΧΪ_____��
��4��ȡһ�κ�̼��0.6%��ϸ��˿�Ƴ�����״��Ͷ��ʢ��������ϡ������ձ��г�ַ�Ӧ���ɹ۲������Ϊ_____�������ձ����������ĺ�ɫ�������仯ѧʽΪC��
���𰸡��ٺ͢� ��˿�к�̼��Խ�ߣ��������������Խ���� 3Fe+2O2 Fe3O4 ��0.6% ��˿�ܽ⣬������ɫ���壬��ɫ��Һ���dz��ɫ
Fe3O4 ��0.6% ��˿�ܽ⣬������ɫ���壬��ɫ��Һ���dz��ɫ
��������
ϸ��˿��������ȼ�����ɺ�ɫ���������������壬����ϡ���ᷴӦ�����Ȼ�������������
[�ռ�֤��]��1���ٺ͢ڵ�����������������ͬ����Ҫ�Ƚ�ϸ��˿ȼ�յľ��ҳ̶��������Ĵ����йأ�Ӧѡ���ʵ��������Ǣٺ͢ڡ���ʵ��ۺܶ͢Աȿ�֪���������������������ϸ��˿�к�̼���Ĺ�ϵ��˿�к�̼��Խ�ߣ��������������Խ���ԡ�
[���������]��2��ϸ��˿��������ȼ�����ɺ�ɫ����������������Ļ�ѧ����ʽΪ3Fe+2O2![]() Fe3O4��
Fe3O4��
��3����˿�к�̼��Ϊ0.6%��̼Ԫ����ȼ�չ��������ɶ�����̼���壬����Ӧ���ɵ��ۻ����������������˳�ȥ���Ӷ������˻������������Ϊ��֤ʵ��ɹ���ѡ�õ���˿�к�̼��Ҫ���Ƶķ�ΧΪ���ڻ����0.6%��
��4������ϡ���ᷴӦ�����Ȼ�������������ȡһ�κ�̼��0.6%��ϸ��˿�Ƴ�����״��Ͷ��ʢ��������ϡ������ձ��г�ַ�Ӧ���ɹ۲������Ϊ��˿�ܽ⣬������ɫ���壬��ɫ��Һ���dz��ɫ�������ձ����������ĺ�ɫ�������仯ѧʽΪC��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��ij��ѧ��ȤС��Ϊ�˲ⶨij��ͭ��ͭ��п�Ͻ���Ʒ��п������������ȡ10����Ʒ�����ձ��У���ȡ60��ϡ��������μ����ձ��У�����ַ�Ӧ��ʵ���������£�
��һ�� | �ڶ��� | ������ | ���Ĵ� | ����� | ������ | |
����ϡ�����������g�� | 10 | 10 | 10 | 10 | 10 | 10 |
ʣ������������g�� | 9.35 | 8.7 | 8.05 | 7.4 | 6.75 | 6.75 |
��1����ͭ��Ʒ��п����������Ϊ���٣���д�����㲽�裬��ͬ��
��2������ϡ�������������Ϊ���٣�
����Ŀ��С���ڼ����������ĺ�����з���һ��˫���������ǩ��ͼ��ʾ���õ�ѧУ��ͬѧ�Ƕ�������õĹ�����ƷҲ�ܺ��棬��������ʦ��ָ���½���������̽����

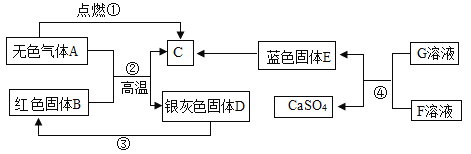
��������⣩���ù���ijɷ���ʲô��
���������룩���ù����п��ܺ���Fe��Fe2O3��CaO��Ca(OH)2��CaCO3��
���������ϣ������Ȼ�����Һ�ڳ����·�����Ӧ�����Ȼ�������
��ʵ��̽����С������˷���һ��
ʵ����� | ʵ������ | ʵ����� |
ȡ������������Թ��У��μ�������______�� | ��������ʧ������ɫ���ݲ������õ�dz��ɫ��Һ�� | ������һ������____��һ������Fe2O3�� |
ͬѧ����ΪС�յķ���һ�����ܵó�һ������Fe2O3�Ľ��ۣ������ǣ�______��������ۺ��������ʵ�������������֤��
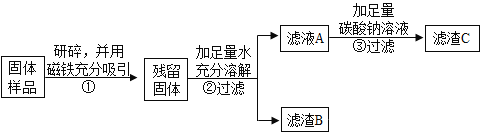
������й����ܽ�ʱ�ų��������ɴ˿����жϹ�����һ������________________�� д���ù����з�Ӧ�Ļ�ѧ����ʽ______________��
ͬѧ�Ƕ�����B�ٽ���̽����
ʵ����� | ʵ������ | ʵ����� |
ȡ����B���Թ��У���������ϡ���ᣬ���ɵ�����ͨ�����ʯ��ˮ | ������٣�__________��ʯ��ˮ����ǡ� | ������һ������CaCO3��Fe2O3�� |
��ʵ����ۣ� �þ��ù�����һ������Fe��Fe2O3��CaO��CaCO3��
����˼�� ʳƷ��װ���ڷ�˫������Ŀ����_________________________________��
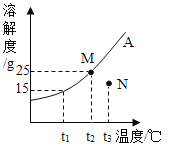
����Ŀ����ʵ��ⶨ�Ȼ��ƺ�������ڲ�ͬ�¶ȵ��ܽ���������±�������˵������ȷ���ǣ�������
�¶ȣ��� | 10 | 20 | 30 | 40 | 50 |
NaCl��g | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 |
KNO3��g | 20.9 | 31.6 | 45.8 | 63.9 | 85.5 |
A.KNO3 ��NaCl���ܽ����ȵ��¶���20�桫30��֮��
B.�������ʵ��ܽ�ȶ����¶ȵ����߶�����
C.��20��ʱ��10gˮ�м���5gNaCl�ɵõ���������Ϊ33.3%��NaCl��Һ
D.�ֱ��������ʵ�100g������Һ��50�潵��10�棬KNO3�����ľ����