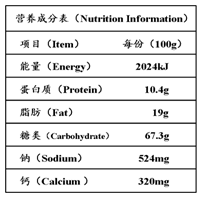
��Ŀ����
С��һ�����ڵ������Ի����

��1��������õ�ȼ����ľ̿����Ȼ�����ƾ�������ƾ����ȡ���Ȼ���е���Ҫ�ɷ��ڿ�����ȼ�յĻ�ѧ����ʽΪ ��
��2������ѡ�IJ�Ʒ�У�ţ��Ƭ����Ƭ���ײˡ����ܲ�������������ѼѪ�ȡ�
�ٲ�Ʒ���ṩ�����ʽ϶���� ����һ�ּ��ɣ��ȡ�
������ѼѪ�к��ж�����Ԫ�أ�������������Ϊ�ḻ�����ʳ��ѼѪ��Ԥ������ �Ĺ�Ч��
��3������ѡ���Թ���ƾ�Ϊȼ�ϵ�С���������ͼ��ʾ����
�۲����Ľṹ�����ֹ���ƾ�����ĵײ�֮����һ���Ŀռ䣬�������һ�¿��ܵ�ԭ���� ��
��4���ڳԻ�������У�С��ϲ����ͭ����ڼ�һ����ʳ�ף�����ʳ���ܷ�ʴ���
��ԭ���� ��

��1��������õ�ȼ����ľ̿����Ȼ�����ƾ�������ƾ����ȡ���Ȼ���е���Ҫ�ɷ��ڿ�����ȼ�յĻ�ѧ����ʽΪ ��
��2������ѡ�IJ�Ʒ�У�ţ��Ƭ����Ƭ���ײˡ����ܲ�������������ѼѪ�ȡ�
�ٲ�Ʒ���ṩ�����ʽ϶���� ����һ�ּ��ɣ��ȡ�
������ѼѪ�к��ж�����Ԫ�أ�������������Ϊ�ḻ�����ʳ��ѼѪ��Ԥ������ �Ĺ�Ч��
��3������ѡ���Թ���ƾ�Ϊȼ�ϵ�С���������ͼ��ʾ����
�۲����Ľṹ�����ֹ���ƾ�����ĵײ�֮����һ���Ŀռ䣬�������һ�¿��ܵ�ԭ���� ��
��4���ڳԻ�������У�С��ϲ����ͭ����ڼ�һ����ʳ�ף�����ʳ���ܷ�ʴ���
��ԭ���� ��
��1��CH4+ 2O2 CO2 + 2H2O
CO2 + 2H2O
��2����ţ��Ƭ������Ƭ������������ѼѪ����ȱ����ƶѪ
��3��ʹ��������ȡ��������ƾ�������Ӵ������ʹȼ�ո����
��4�����ܣ���Ϊͭ���ڽ����˳�������ĺ��治����ᷴӦ
 CO2 + 2H2O
CO2 + 2H2O��2����ţ��Ƭ������Ƭ������������ѼѪ����ȱ����ƶѪ
��3��ʹ��������ȡ��������ƾ�������Ӵ������ʹȼ�ո����
��4�����ܣ���Ϊͭ���ڽ����˳�������ĺ��治����ᷴӦ
��������1������ȼ�յĻ�ѧ����ʽCH4+2O2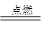 CO2+2H2O��
CO2+2H2O��
��2����Ʒ���ṩ�����ʽ϶����ţ��Ƭ����Ƭ�ȣ�����ѼѪ�к��ж�����Ԫ��������������Ϊ�ḻ�����ʳ��ѼѪ��Ԥ������ƶѪ��
��3���۲����Ľṹ�����ֹ���ƾ�����ĵײ�֮����һ���Ŀռ䣬�¿��ܵ�ԭ����ȼ����Ҫ����Ŀ�����
��4����ͭ����ڼ�һ����ʳ�ף����ܸ�ʴ�������Ϊͭ�������Ӧ�û���������
��𣺽⣺
��1����Ȼ���е���Ҫ�ɷ��ڿ�����ȼ�յĻ�ѧ����ʽCH4+2O2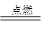 CO2+2H2O��
CO2+2H2O��
��2����Ʒ���ṩ�����ʽ϶����ţ��Ƭ����Ƭ�ȣ�����ѼѪ�к��ж�����Ԫ��������������Ϊ�ḻ�����ʳ��ѼѪ��Ԥ������ƶѪ��
��3���۲����Ľṹ�����ֹ���ƾ�����ĵײ�֮����һ���Ŀռ䣬�������һ�¿��ܵ�ԭ����ȼ����Ҫ����Ŀ�����
��4��С��ϲ����ͭ����ڼ�һ����ʳ�ף�����ʳ�ײ��ܸ�ʴ�������Ϊͭ�������Ӧ�û���������
�ʴ�Ϊ����1��CH4+2O2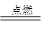 CO2+2H2O��2��ţ��Ƭ��ƶѪ��3��ȼ����Ҫ����Ŀ�����4�����ܡ�ͭ�������Ӧ�û���������
CO2+2H2O��2��ţ��Ƭ��ƶѪ��3��ȼ����Ҫ����Ŀ�����4�����ܡ�ͭ�������Ӧ�û���������
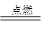 CO2+2H2O��
CO2+2H2O����2����Ʒ���ṩ�����ʽ϶����ţ��Ƭ����Ƭ�ȣ�����ѼѪ�к��ж�����Ԫ��������������Ϊ�ḻ�����ʳ��ѼѪ��Ԥ������ƶѪ��
��3���۲����Ľṹ�����ֹ���ƾ�����ĵײ�֮����һ���Ŀռ䣬�¿��ܵ�ԭ����ȼ����Ҫ����Ŀ�����
��4����ͭ����ڼ�һ����ʳ�ף����ܸ�ʴ�������Ϊͭ�������Ӧ�û���������
��𣺽⣺
��1����Ȼ���е���Ҫ�ɷ��ڿ�����ȼ�յĻ�ѧ����ʽCH4+2O2
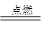 CO2+2H2O��
CO2+2H2O����2����Ʒ���ṩ�����ʽ϶����ţ��Ƭ����Ƭ�ȣ�����ѼѪ�к��ж�����Ԫ��������������Ϊ�ḻ�����ʳ��ѼѪ��Ԥ������ƶѪ��
��3���۲����Ľṹ�����ֹ���ƾ�����ĵײ�֮����һ���Ŀռ䣬�������һ�¿��ܵ�ԭ����ȼ����Ҫ����Ŀ�����
��4��С��ϲ����ͭ����ڼ�һ����ʳ�ף�����ʳ�ײ��ܸ�ʴ�������Ϊͭ�������Ӧ�û���������
�ʴ�Ϊ����1��CH4+2O2
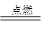 CO2+2H2O��2��ţ��Ƭ��ƶѪ��3��ȼ����Ҫ����Ŀ�����4�����ܡ�ͭ�������Ӧ�û���������
CO2+2H2O��2��ţ��Ƭ��ƶѪ��3��ȼ����Ҫ����Ŀ�����4�����ܡ�ͭ�������Ӧ�û���������
��ϰ��ϵ�д�
�����Ŀ