��Ŀ����
����Ŀ����ѧ����С���ͬѧ���������ƺ�ϡ����̽���кͷ�Ӧ�������������̽����
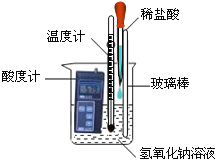
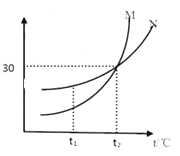
(1)����ͬѧȡ����������������Һ���ձ��У�����ȼƲ����Һ��pH ��ͬʱ����һ֧�¶ȼƣ�������Һ���¶�(��ͼ��ʾ)���ý�ͷ�ι���ȡϡ���ᣬ��μ���ʢ������������Һ���ձ��У��ߵμӱ߽��衣��ʱ�ɹ۲쵽�������ǣ���ȼ�����ʾ��������______(ѡ����������������С������������)���¶ȼ�����ʾ�����������ߣ��ɴ˿�֪����ͼ���кͷ�Ӧ����___(ѡ��������������������)��Ӧ��
(2)�����ͬѧ���ù�������������ϡ���ᷴӦ��̽���кͷ�Ӧ�������ı仯������� ��Ϊ�÷���___(ѡ����������������������)��ԭ��___��
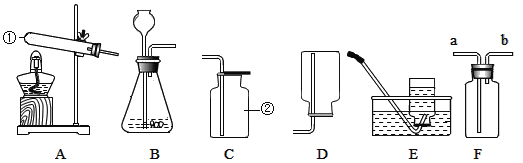
(3)����ͬѧ������һƿ����������Һ����ʵ��ʱ�������ձ��еμ�ϡ����ʱ������������������ð����С��ͬѧ������һ����������ۣ�һ����Ϊ��ԭ�����������������Һ�Ѿ����ʣ��û�ѧ����ʽ��ʾ���������Ʊ��ʵ�ԭ��___��Ϊ�˳�ȥ����������Һ�б��ʲ�����̼���ƣ�����Ϊ��ѡ�õ�������_____(����ĸ)
A��ϡ���� B������������Һ C���Ȼ�����Һ D���Ȼ�����Һ
(4)����ͬѧ������̪��Һ���ж�����������Һ����ϡ���ᷢ���кͷ�Ӧ�����ձ��м�������������Һ�μ��η�̪��Һ��Ȼ�����ϡ���ᣬ�����ҺΪ��ɫ��С����Ϊ��Һ�����ԡ�С����Ϊ��һ����С��ͬѧ�����ʵ�������֤�������С��Ľ���,��д��С����Ƶ�ʵ��(Ҫ��д��������������)��_______
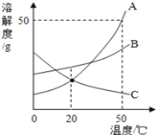
(5)����ͬѧ��������ͬѧʵ�����õ�����������Һ�����ⶨ����������ȡ��100g����Һ����������εμ�������������Ϊ14.6%��ϡ���ᣬ����ϡ������������������������֮��Ĺ�ϵ��ͼ��ʾ���ݴ��ƲⲢ���㣺
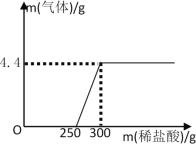
�ٸ�����������Һ�ı��������________(ѡ����ȫ���ʡ����ֱ���)
�ڸ�����������Һ��̼���Ƶ�����Ϊ_____g��
�۵�����300gϡ�����ַ�Ӧ��������Һ��������������_____��(�����ȷ��0.1%��Ҫ��д���������)
���𰸡���С ���� ������ ����������������ˮ���ȣ����жϷ�Ӧ�Ƿ���� 2NaOH + CO2 == Na2CO3 + H2O B ȡ��������̼���(��μ�̼������Һ)�������ݲ������������������Һ�����ԡ�(�����Լ��������ɣ��������ɫʯ����Һ��pH��ֽ�ȵ�) ���ֱ��� 10.6 17.7%
��������
(1)��ͼʾ��֪����ȼƷ���������������Һ�У���������������Һ����μ���ϡ����ʱ�������кͷ�Ӧ����Һ�ļ��Լ�����pH��С���¶ȼ�����ʾ�����������ߣ��ɴ˿�֪����ͼ���кͷ�Ӧ���ڷ��ȷ�Ӧ��
(2)�����ͬѧ���ù�������������ϡ���ᷴӦ��̽���кͷ�Ӧ�������ı仯������÷�����������ԭ���ǹ���������������ˮ���ȣ����жϷ�Ӧ�Ƿ���ȡ�
(3)����������Һ������Ӵ�����������еĶ�����̼��Ӧ����̼���ƣ�2NaOH+CO2== Na2CO3+H2O��ѡ�ó����Լ���Ҫ��ѭ����ԭ��һ�Ǽ�����Լ�ֻ�������ʷ�Ӧ�����Ƿ�Ӧ���������µ�����Na2CO3+Ca(OH)2==CaCO3��+2NaOH����ѡB��
(4)��̪��Һ�����ԡ�������Һ������ɫ����Ϊ��ɫ�����ձ��м�������������Һ�μ��η�̪��Һ��Ȼ�����ϡ���ᣬ�����ҺΪ��ɫ����Һ���������ԣ�Ҳ���������ԡ�ȡ��������̼���(��μ�̼������Һ)�������ݲ������������������Һ�����ԣ��������ɫʯ����Һ����ҺΪ��ɫ������Һ�����Ի���pH��ֽ����Һ��pHֵ��pH<7����Һ�����ԡ���
(5)�ٸ���ͼ���֪�����������ƺ�̼���ƵĻ����Һ�м���ϡ���ᣬ��ʼû�в������壬����ϡ���������������Ʒ����кͷ�Ӧ�����������Ʒ�Ӧ���Ժ�ϡ�������̼���Ʒ�Ӧ�������壬��������������Һ���ֱ��ʡ��ڸ���ͼ���֪��������4.4g�����Ƕ�����̼���������������Һ��̼���Ƶ�����Ϊx��

![]() =
=![]() �����x=10.6g
�����x=10.6g
��Na2CO3+2HCl==2NaCl+H2O+CO2����NaOH+HCl==NaCl+H2O
����HCl-------NaCl��
��ͼ���֪��������300gϡ���ᣬϡ����������Һǡ�÷�Ӧ��������NaCl������Ϊy��

![]() =
=![]() �����y=70.2g
�����y=70.2g
����300gϡ���ᷴӦ����Һ������Ϊ��100g+300g-4.4g= 395.6g
������Һ��������������Ϊ��![]() ��100%=17.7%
��100%=17.7%