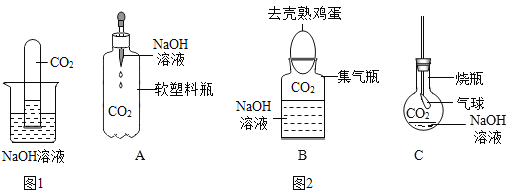
��Ŀ����
����Ŀ��ijͬѧȡ�����ƺ�����þ��������26.2g�������м���219.6gˮ��ʹ����ȫ�ܽ����Ƴ���Һ����200gһ��������������������������Һƽ���ֳ�5�ȷݣ����μ��뵽������Һ�У����ɳ������������������������Һ��������ϵ���±���
����������Һ������/g | ��һ�μ���40 | �ڶ��μ���40 | �����μ���40 | ���Ĵμ���40 | ����μ���40 |
������������/g | 1.45 | 2.9 | m | 5.8 | 5.8 |
���㣺��1��m��ֵΪ_____��
��2����������������þ��������
��3��ǡ����ȫ��Ӧʱ�����ò�������Һ�����ʵ�����������
���𰸡���1��4.35����2��12g����3��7.1%
��������
����þ��Һ������������Һ��Ӧ����������þ��������������Һ��
��1���ɱ�������ɵã�ÿ����40g����������Һ��Ӧ����1.45g���������Ե����μ���40g��������ʱ�����ɳ���������=![]() ������m��ֵΪ4.35�����4.35��
������m��ֵΪ4.35�����4.35��
��2����3���⣺���������������þ������Ϊ![]() ��ǡ�÷�Ӧʱ���������Ƶ�����Ϊ
��ǡ�÷�Ӧʱ���������Ƶ�����Ϊ![]()
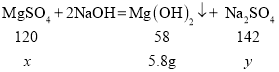
![]() ��
��![]()
![]() ��
��![]()
��ԭ�����������þ����Ϊ12g��
������ҺΪ��������Һ����������Һ����������Ϊ��![]() ��
��
ǡ����ȫ��Ӧʱ����������������Һ������Ϊ![]() ��������Һ������
��������Һ������![]()
���ò�������������Һ��������������Ϊ![]()
�𣺣�2����������������þ������Ϊ12g��
��3��ǡ����ȫ��Ӧʱ�����ò�������Һ�����ʵ���������7.1%��

 �ܿ�����ĩ��̾�ϵ�д�
�ܿ�����ĩ��̾�ϵ�д�����Ŀ��ij����������һ�����������ж��¼�������������ۿ���ʳ��������ζ���������ƺ�ʳ�ε��й��������£�
�� Ŀ | �������� | �Ȼ��� |
ˮ���� | ���ܣ���15��ʱ�ܽ��Ϊ81.5g | ���ܣ���15��ʱ�ܽ��Ϊ35.8g |
�� �� | 271�� | 801�� |
�� �� | 320���ֽ⣬�ų��г�ζ������ | 1413�� |
��ϡ�������� | �ų�����ɫ������ | ��Ӧ |
(1)�����ϱ�������д���������Ƶ������������ʣ���_____����________��
(2)�����������Ƶķ��������ǣ�_____��