��Ŀ����
����Ŀ�����������������У�ѡ�������ȡ������̼��װ�ã����ж�����̼����ȡ������̽����

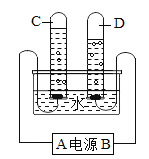
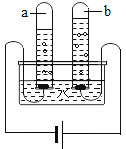
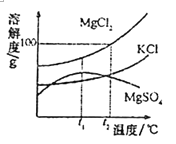
(1)ָ��ͼ�����������ƣ���_____________��
(2)��ȡ���ռ�������̼��ѡ����ͼ�����е�_____________(�����)����Ӧ�Ļ�ѧ����ʽΪ__________________________��
(3)�ռ���һƿ������̼���������̼�Ƿ��ռ����ķ�����__________________________��
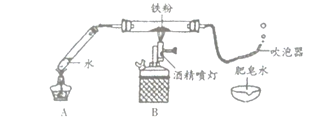
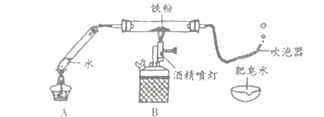
(4)Ϊ��̽��������̼����ˮ������Ӧ���ɾ������Ե����ʣ��ס�����ͬѧ�ֱ������̽��ʵ��ķ���(��ͼ�ס�����ʾ)����ʵ�顣ͼ�Ҳ��õ��Ķ仨Ϊ��ʯ����ҺȾ����ɫ�ĸ����ֽ����

��������ס���ͬѧһ�����̽����
������ | (��) | (��) | (��) | (��) |
���� | ���ɫ | ����ɫ | ����ɫ | ���ɫ |
����ͼ�ס�����CO2ʹʯ����Һ���ɫ��ԭ��_______________��
������Ϊ�ס�����λͬѧ��ʵ��̽��������������˵��������ɣ�_______________________��
���𰸡���ƿ �ڢۢܢ� CaCO3+2HCl=CaCl2+CO2��+ H2O ��ȼ�ŵ�ľ��ƽ����ƿ�ڣ���ľ��Ϩ�������� ������̼��ˮ��Ӧ������̼�� ��ͬѧ������������ͬѧͨ���Ա�ʵ�飬�ų���ˮ������Ķ�����̼����ʹ��ɫʯ���죬�Ӷ�֤���˶�����̼��ˮ��������������ʣ���ͬѧ����֤��
��������
(1) ͼ�������ڵ����ƣ���ƿ��
(2)ʵ������һ����ʯ��ʯ��ϡ������ȡ������̼����ѡ�ù�Һ������װ�ã�������̼���ܶȱȿ�����������ˮ����ˮ��Ӧ����ֻ���������ſ���������ѡ�õ�����Ϊ�ڢۢܢߣ���Ӧ�Ļ�ѧ����ʽΪ��CaCO3+2HCl=CaCl2+CO2��+ H2O��
(3)������̼��ȼ��Ҳ��֧��ȼ�գ��ʿ���ȼ�ŵ�ľ�����飬����Ӧ����ƿ�ڣ��ʼ��鷽��Ϊ����ȼ�ŵ�ľ��ƽ����ƿ�ڣ���ľ��Ϩ����������
(4)��ͨ���Ա������֪��CO2ʹʯ����Һ���ɫ��ԭ���ǣ�������̼��ˮ��Ӧ������̼�
����ͬѧ������������ͬѧͨ���Ա�ʵ�飬֤����ˮ������Ķ�����̼����ʹ��ɫʯ���죬�Ӷ�֤���˶�����̼��ˮ��������������ʣ���ͬѧ����֤����

 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�����Ŀ��ʵ����ϣ�����ʦ��ʧȥ��ǩ��һ�ֹ���A��һ��Һ��B��Ϻ���������ð����������⣺���������������壿
����һ��������̼��
�������������������_______���û�ѧ����ʽ��ʾ��
������_______��������_______���û�ѧ����ʽ��ʾ��
ʵ�鲽�� | ʵ�������뻯ѧ����ʽ | ʵ����� |
����һ���ֱ�ȡ����ҩƷ������һ֧�Թ��У�����ȼ�ŵ�ľ�������Թ��С� | ����_______�� | ����һ��ȷ |
��������_______�� | ����_______����ѧ����ʽ��_______�� |
ʵ�鷴˼������A��Һ��B��Ϻ���ܷ�����Ӧ�Ļ�ѧ����ʽ��_______��