��Ŀ����
����Ŀ��4�� 23 �գ���ף�й������ž��������� 70 ���꺣���ı�����ൺ������������У��й���������ն�ˣ��������������������ź���ĸ��Ҳ�����˼��ģ���ĸ�ķ��мװ壬���ܳ��ܽ��ػ���ʱ������ǿ�ҳ����Ħ����Ҳ�ܳ��ܽ��ػ���β�����䣬���ֿܵ���ʪ�����ĺ������������ʴ��������ѧ֪ʶ��ա�
��1�����캽ĸ���мװ��õ����ѺϽ���Ҫ�����Ľ����ѣ��������ܶ�С���۵�ߡ��� ��ʴ���������ܣ�����Ϊ��δ������������ҵ������һ�ַ�ӦΪ��TiF4+2H2SO4![]() TiO2+4HF+2X������ X �Ļ�ѧʽΪ_______________��
TiO2+4HF+2X������ X �Ļ�ѧʽΪ_______________��
��2����ĸ�����Ϳ�ϸ��ǣ���Ϊ�˷�ֹ����������______________�Ӵ�����ʴ��
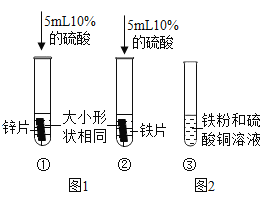
��3���Ͻ��Ӧ�����������������չ����������������ʹ�õĺϽ������_____������ĸ��ţ���
A���Ͻ� B���Ͻ� Cͭ�Ͻ�
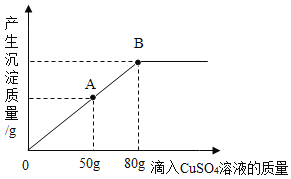
��4���� 7g ��п�Ͻ��в��ϼ���һ����������������ϡ���ᣨ�����Ѳ���ϡ���ᷴӦ���� ����ϡ�������������������Ĺ�ϵ��ͼ��ʾ������㣺
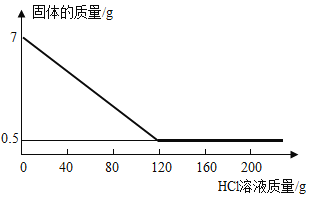
�ټ���һ����������������ϡ����Ĺ����г��ֵ�һ��������___________��
�ںϽ���п������Ϊ_________g��
�۵�����ϡ���� 120g ʱ�����ˣ���������Һ��������������____________������������ 0.1%����
���𰸡�SO3 ˮ������ C �����������������ܽ� 6.5 10.8%
��������
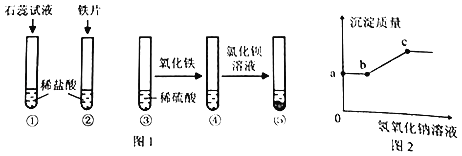
��1�����������غ㶨�ɣ���ѧ��Ӧǰ��ԭ�ӵ��������Ŀ���䣬���![]() ���ɵ�X�Ļ�ѧʽΪ��SO3��
���ɵ�X�Ļ�ѧʽΪ��SO3��
��2����ĸ�����Ϳ�ϸ��ǣ���Ϊ�˷�ֹ����������������ˮ�Ӵ�����ʴ��
��3����������ʹ�õĺϽ�����ǣ�ͭ�Ͻ𡣹�ѡC��
��4���ټ���ϡ���ᣬп��ϡ���ᷴӦ�����Ȼ�п���������ʼ���һ����������������ϡ����Ĺ����г��ֵ�һ�������ǣ������ݲ�������������ܽ⣻
�ںϽ���п������Ϊ��7g-0.5g=6.5g��
�۽⣺��п��ȫ��Ӧ�����Ȼ�п������Ϊx����������������Ϊy
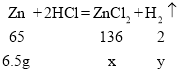
![]() x=13.6g
x=13.6g
![]() y=0.2g
y=0.2g
������Һ����������������![]()
��������Һ��������������Ϊ��10.8%��

 ���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д�
���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д� ��ٽ������½������������ϵ�д�
��ٽ������½������������ϵ�д�