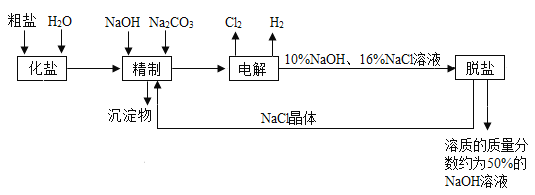
��Ŀ����
����Ŀ��һ��Ļ�ѧѧϰ�����ǶԻ�ѧ��Ӧ����һ������ʶ��
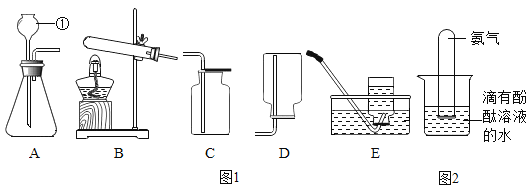
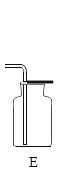
(1)���ػ�ѧ��Ӧ�Ŀ�������ʵ������ȡ�����������ö���������_____________���ӿ췴Ӧ���У��䷴Ӧ�Ļ�ѧ����ʽΪ_________________________��
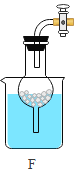
(2)�жϻ�ѧ��Ӧ�ķ�������ʵ������ȡ������̼������ͨ��________________�����жϷ�Ӧ�ķ������䷴Ӧ�Ļ�ѧ����ʽΪ_____________________________��
(3)��ʶ��ѧ��Ӧ��ʵ�ʡ�������������������Һ�ķ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ_________________________���䷴Ӧ��ʵ����_________________________��
(4)��ѧ��Ӧ�������������仯���練Ӧ:______________________(�û�ѧ����ʽ��ʾ)�� ���������ų���
(5)��ѧ��Ӧ���������غ㶨�ɡ���4g������16g������ַ�Ӧ������ˮ������Ϊ___ g��
���𰸡����� 2H2O2![]() 2H2O+O2����
2H2O+O2����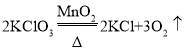 �������ݵIJ��� CaCO3+2HCl��CaCl2+H2O+CO2�� NaOH+HCl��NaCl+H2O �����Ӻ����������ӽ������ˮ C+O2
�������ݵIJ��� CaCO3+2HCl��CaCl2+H2O+CO2�� NaOH+HCl��NaCl+H2O �����Ӻ����������ӽ������ˮ C+O2![]() CO2 18
CO2 18
��������
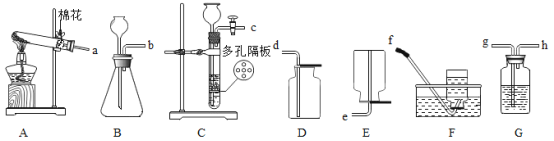
��1�����������ڶ������̵Ĵ�������ˮ��������������ڶ������̵Ĵ��¼��������Ȼ��غ�����������ʽΪ2H2O2![]() 2H2O+O2����
2H2O+O2����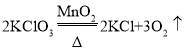 �������������������
�������������������
��2��̼��ƺ�ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼������ʽΪ��CaCO3+2HCl��CaCl2+H2O+CO2����ͨ���ж��������ݵIJ����жϷ�Ӧ�Ƿ�����
��3���������ƺ�ϡ���ᷴӦ�����Ȼ��ƺ�ˮ������ʽΪ��NaOH+HCl��NaCl+H2O���кͷ�Ӧ��ʵ�������е������Ӻͼ��е����������ӽ������ˮ�ķ�Ӧ��
��4����ѧ��Ӧ�������������仯��������ȼ�ա��к͡�������û���Ӧ���Ƿ��ȷ�Ӧ����C+O2![]() CO2��
CO2��
��5��������������Ӧ�ķ���ʽΪ��2H2+O2![]() 2H2O���ɷ���ʽ�ɵ�����������ǡ����ȫ��Ӧ��������Ϊ4��32��1��8�����4g������16g������ַ�Ӧ��ֻ������2g����������������ˮ������Ϊ18g��
2H2O���ɷ���ʽ�ɵ�����������ǡ����ȫ��Ӧ��������Ϊ4��32��1��8�����4g������16g������ַ�Ӧ��ֻ������2g����������������ˮ������Ϊ18g��

 ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д� ����С��ҵϵ�д�
����С��ҵϵ�д� �Ƹ�С״Ԫ����������ϰ��ϵ�д�
�Ƹ�С״Ԫ����������ϰ��ϵ�д� �ɹ�ѵ���ƻ�ϵ�д�
�ɹ�ѵ���ƻ�ϵ�д� ����ѵ����ֱͨ�п�����ϵ�д�
����ѵ����ֱͨ�п�����ϵ�д� һ���㶨ϵ�д�
һ���㶨ϵ�д�����Ŀ������ͭ���峣����ɱ�桢ɱ�����÷�ͭ�ϣ�������������������ͭ�����������ͼ��ʾ����ش��������⣮
![]()
��1������ a ��������_____��
��2����ͭ���м��������Լ� X ��Ŀ����_____��
��3����ɫ���� B ���Լ� X �ķ�Ӧ��ѧ����ʽ��______��
��4��ʢ������ͭ��Һ����������������ԭ���ǣ��û�ѧ����ʽ���ͣ�______��
��5����������ͭ���ܽ�ȱ�����ʾ���������ͭ��Һ����ͨ������Ũ����_____�����ˡ�ϴ�ӡ�����ķ����õ�����ͭ���塣
0�� | 10�� | 20�� | 30�� | 40�� | 60�� | 80�� | 100�� | |
����ͭ/g | 23.1 | 27.5 | 32 | 37.8 | 44.6 | 61.8 | 83.8 | 114 |