��Ŀ����
ʵ����������������п������״ʯ��ʯ��ϡ��������ҩƷ��ľ�顢�������ʵ����Ʒ����ش��������⣺
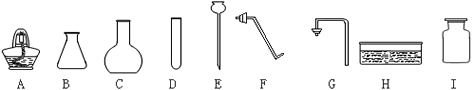
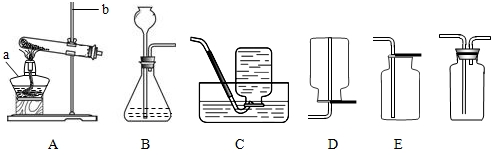
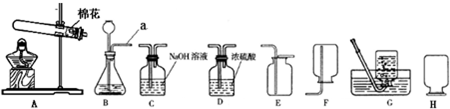
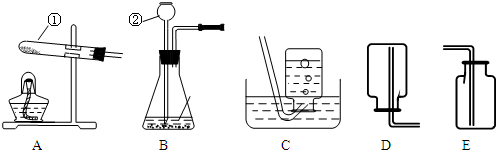
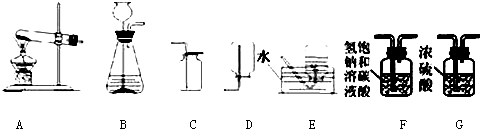
(1)�����������A_________��B_________�� E__________�� H_________��
(2)��������������ҩƷ����ȡ�����壬�û�ѧ����ʽ��ʾ��ȡ������Ļ�ѧ��Ӧԭ��________________________�� ��ȡ��������ѡ���������(���������)__________________��
(3)��ʵ����������������ҩƷΪ________��������ȡO2 ����ȡ��������ѡ���������(���������)__________________��
(4)�뻭��O2 ���ռ�װ��ͼ��
(2)��������������ҩƷ����ȡ�����壬�û�ѧ����ʽ��ʾ��ȡ������Ļ�ѧ��Ӧԭ��________________________�� ��ȡ��������ѡ���������(���������)__________________��
(3)��ʵ����������������ҩƷΪ________��������ȡO2 ����ȡ��������ѡ���������(���������)__________________��
(4)�뻭��O2 ���ռ�װ��ͼ��
��1��A�ƾ��ƣ�B��ƿ��E����©����Hˮ��
��2��CaCO3+2HCl===CaCl2+H2O+CO2������Zn+2HCl===ZnCl2+H2������ BEGI
���𰸲�Ψһ��
��3��������أ�ADFHI ���𰸲�Ψһ��
��4��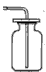
��2��CaCO3+2HCl===CaCl2+H2O+CO2������Zn+2HCl===ZnCl2+H2������ BEGI
���𰸲�Ψһ��
��3��������أ�ADFHI ���𰸲�Ψһ��
��4��


��ϰ��ϵ�д�
 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
�����Ŀ