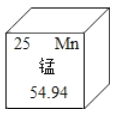
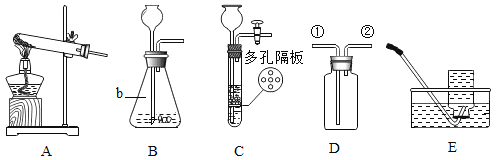
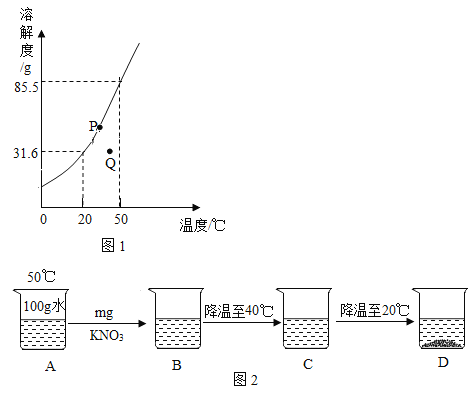
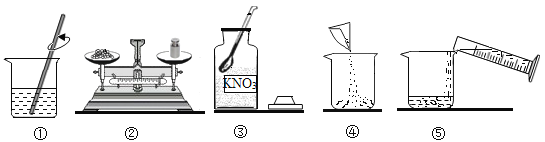
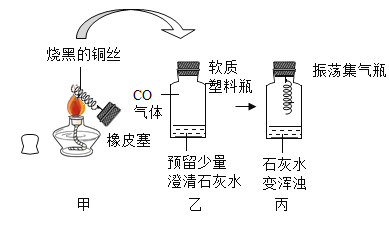
��Ŀ����
����Ŀ�����������������벻����ѧ��
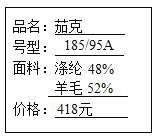
��1����ͼ��ijƷ�Ʒ�װ��ǩ�ϵIJ������ݣ�������������Ȼ��ά����_________�������г���_________�ķ�������ϳ���ά����Ȼ��ά��
��2�������������________________����ȥˮ���е�ˮ��[ˮ������Ҫ�ɷ���CaCO3��Mg(OH)2]��
��3������ϴ�ྫ��ȥ�;��ϵ����ۣ�����Ϊϴ�ྫ�����۾���___________���á�
��4������������ζ�������ͣ�������ͬѧ���Ż�û���ǵĿ��֣��ܻؽ��ң���ƿ�Ǻ���ˮ��ӿ���������û�ѧ����ʽ������ԭ��:____________��
��5������ʳƷ�и�����Ӫ������Ϊ�����ṩ��������___________������ţ���
a���� b��Ȫˮ c�߲� dţ���
���𰸡���ë ���� ʳ�� �黯 H2CO3=H2O+CO2�� ad
��������
��1��ͨ��ͼʾ���Կ������������У���ë������Ȼ��ά�����������л��ϳɲ��ϡ���ë�ijɷ��ǵ����ʣ���ȼ���ս���ë����ζ������������û���ս���ë����ζ���ʿ���ͨ�����յķ�������ϳ���ά����Ȼ��ά��
��2��ˮ������Ҫ�ɷ���CaCO3��Mg(OH)2�������ᷴӦ���������ó����е�ʳ����ϴˮ���е�ˮ����
��3��ϴ�Ӽ������黯���ã��ܳ�ȥ�����ϵ����ۣ�
��4����ˮ�ǽ�������̼�����ѹ֮���Ƴɵģ�����ˮƿ�ǣ�ѹǿ��С��������̼���ܽ�ȼ�С�����д������ݴ�ƿ���ݳ���˵����������ܽ����ѹǿ�ļ�С����С����ѹǿ�����������Ӧ�Ļ�ѧ����ʽ��H2CO3=H2O+CO2����
��5�����ࡢ֬���������ʶ���Ϊ�����ṩ�����������и����е����ʣ���Ϊ�����ṩ�������߲��и�����ά���أ�����Ϊ�����ṩ������ţ����и����е����ʣ���Ϊ�����ṩ��������Ȫˮ����Ϊ�����ṩ��������ѡad��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�