��Ŀ����
����Ŀ����2019����꼶��ѧʵ��������ܿ����У�С��ͬѧ�鵽����������̽����ͼ�֮����кͷ�Ӧ��������ʢ������������Һ���ձ��л����μ�ϡ���ᣬһ������������Ǽ�ָʾ���ˣ��㲹�Ӽ�����ɫ��̪��Һ�����裬�۲쵽��Һ����ɫ���������ó������ǡ����ȫ�к����Ľ��ۡ�
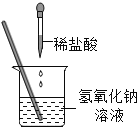
��1�����кͷ�Ӧ�Ļ�ѧ����ʽ��__________��
��2����ʦ����С�ϵ������ǽ��۲�ȷ�������С�Ϸ�����ʦ�������ǣ�_____��
��3�������������һ��ʵ�飬̽��С��ʵ����ձ��е���Һ�Ƿ���ǡ����ȫ�к�����
������
ʵ�鲽�� | ʵ������ | ʵ����� |
ȡ������Һ���Թ��У�_______ | ______ | ϡ������� |
______ | _______ |
��4����˼���кͷ�Ӧ�����У����������Ե�ʵ��������ȷ����Ӧ�Ƿ�������Ӧ���еij̶ȣ���ˣ���Ҫ����һ���ķ������жϡ��������������⣬��������_________���м��顣
���𰸡�NaOH+HCl=NaCl+H2O ������μӷ�̪Ҳ����ɫ������ɫ����������� ������˿ �������� û������ �������ƺ�ϡ����ǡ����ȫ�к� ��pHֵ
��������
��1���������ƺ����ᷴӦ�����Ȼ��ƺ�ˮ����ѧ����ʽΪNaOH+HCl=NaCl+H2O�����NaOH+HCl=NaCl+H2O��
��2��������μӷ�̪Ҳ����ɫ������ɫ����������������������μӷ�̪Ҳ����ɫ������ɫ�������������
��3��ȡ������Һ���Թ��У�������˿����������˵��ϡ���������û������˵���������ƺ�ϡ����ǡ����ȫ�к͡�
��4���������������⣬�������òⶨpH���鷴Ӧ���г̶ȣ������pHֵ��

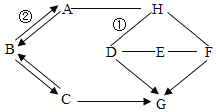
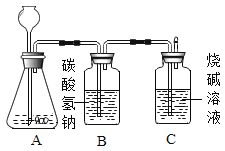
����Ŀ����ѧ��ȤС��Ϊ�ⶨ����ʯ��̼��Ƶĺ���������ͼ��ʾ:��������ϡ������뵽20g����ʯ��(�����ɷֲ������ᷴӦ)���Ѳ�����CO2�������������ռ���Һ���գ�ͬʱ����Cƿ�ռ���Һ���ӵ�������������±���ʾ:
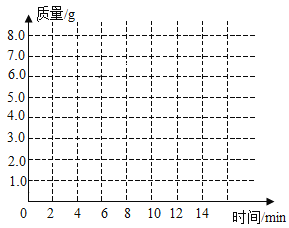
ʱ��/�� | 0 | 2 | 4 | 6 | 8 | 10 | 12 |
C��������/C | 0 | 3.0 | 5.0 | 6.0 | 6.6 | x | 6.6 |
(1)��1���ϱ��У���10����ʱ��x=___;
(2)��2���������ʯ��Ʒ��̼��Ƶ���������(д���������) ___
(3)��3��������ͼ������ֽ�ϣ���ʱ��Ϊ�����꣬�Բ���CO2���������Ϊ�����꣬�����ܹ��������������������ʱ��仯���ɵĹ�ϵ����: ___;
��4��B����װҩƷΪ����̼��������Һ�����������տ��ܻӷ���HCl���壬����Ϊû��B�����������____.(�ƫ�ߡ���ƫ�͡�)��
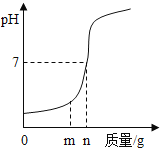
����Ŀ���ձ���ʢ��x��Һ����μ��루��ͨ�룩y���ʣ��ձ���Һ���pH�仯��ͼ��ʾ������ϸñ仯��һ�������ǣ�������
x | y | |
A | NaOH��Һ | ϡHCl�������� |
B | Ca��OH��2 | CO2�������� |
C | ϡH2SO4 | Ba��NO3��2��Һ |
D | ϡHCl | H2O |
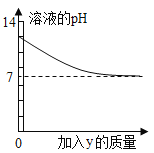
A.AB.BC.CD.D