��Ŀ����
����Ŀ��Ϊ�˲ⶨij������������������ijͬѧ����������ʵ�飺ȡ10.0g����ĸ���Ʒ�����ձ��У���122.5gϡ�����5�μ�����ձ��У���ַ�Ӧ��(�������ʲ�����ˮ��Ҳ�����ᷴӦ) �����ʣ�������������ݼ�¼���£�
���� | 1 | 2 | 3 | 4 | 5 |
����ϡ���������/g | 24.5 | 24.5 | 24.5 | 24.5 | 24.5 |
ʣ����������/g | 7.9 | 5.8 | 3.7 | a | 0.2 |
����㣺
(1)H2SO4������Ԫ�ص�������Ϊ (�����������)��
(2)������a��ֵΪ ��
(3)�ø���Ʒ��������������Ϊ ��
(4)����ϡ�������������������
���𰸡���1��1:2����2��1.6����3��98%����4��15%
��������
(1) H2SO4������Ԫ�ص�������=32����4��16��=1 : 2��
(2)���ݱ��е����ݷ�����ǰ3�μ���24.5gϡ���ᣬʣ����������ÿ�μ���2.1g��˵��24.5gϡ������2.1g ����ȫ��Ӧ�����Ե�4�μ���ϡ�����ǣ�Ҳ����2.1g������a=1.6��
(3)���ݱ������ݷ���������5�μ���24.5gϡ����ʱ����������ֻ������1.4g��˵�����ѷ�Ӧ��ȫ��ʣ���0.2gΪ���ʣ�����10.0g������������Ϊ9.8g��������������![]() ��
��
(4)����(2)�з�����֪��ÿ24.5gϡ������2.1g ����ȫ��Ӧ��������24.5 gϡ���������ʵ�����Ϊx��
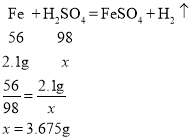
����ϡ�����������������![]()

 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�����Ŀ��ijѧУ��ѧϰС��Ե��ص�ʯ��ʯ�������е��飬�ⶨʯ��ʯ��̼��Ƶ��������������õķ�������:ȡ��ʯ��ʯ��Ʒ16g����100 gϡ�����5�μ��룬���������������ݼ��±�����֪ʯ��ʯ��Ʒ�����ʲ�����ˮ������ϡ���ᷴӦ��������ϡ���������ͷ�Ӧ��ʣ�����������¼����:
����ϡ���������/g | 20 | 20 | 20 | 20 | 20 |
ʵ���ʣ����������/g | 13 | 10 | 7 | 6 | m |
��ش�:
��1���ϱ���m����ֵΪ ��
��2��ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ���٣�
��3���ڵ�5��ʵ������Һ�У��ټ��������ĸ�ʯ��ʯ��Ʒ��ĩ����ȫ��Ӧ����ˣ���������Һ����������������������ʵ���������Һ��ʧ���Բ��ƣ�
��4������16g��Ʒ�м���ϡ�����������ʣ������������ϵ��ʾ��ͼ ������д��������̡���
��5����һ�μ���20 g����ķ�Ӧ����Ϊ��1���ڶ��μ���20g����ķ�Ӧ����Ϊ��2������1 ��2��ѡ����>����<������=������