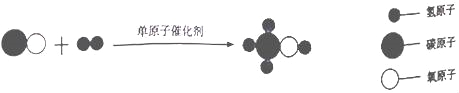
��Ŀ����
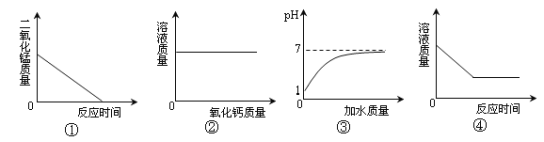
����Ŀ��Cu�CZn�Ͻ�����ڹ���Ʒ��������ij��ѧ��ȤС���ͬѧΪ�˲ⶨijͭп�Ͻ���Ʒ����ɣ�ȡ20����Ʒ���ձ��У������з�5�μ�����ͬ����������ϡ���ᣬʹ֮��ַ�Ӧ��ÿ������ϡ�����������Ϊ20 g��ʣ������������¼���±���
ʵ����� | ��1�� | ��2�� | ��3�� | ��4�� | ��5�� |
��Ӧ��ʣ������������g�� | 17.4 | 14.8 | 12.2 | 10.8 | 10.8 |
�Իش��������⣺
��1��Cu�CZn�Ͻ�����___________���ϣ������������л��ϳɡ�����
��2������Cu�CZn�Ͻ���ȫ��Ӧʱ��������������___________��������������2λС����
���𰸡����� 0.28g
��������
��1���������ϰ��������ͺϽ�Cu-Zn�Ͻ����ڽ������ϡ����������
��2���μӷ�Ӧ��п������Ϊ20g-10.8g=9.2g������ȫ��Ӧʱ��������������Ϊx��
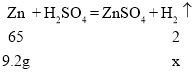
![]()
x��0.28g��
Cu-Zn�Ͻ���ȫ��Ӧʱ��������������Ϊ0.28g�����0.28g��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ��ij�о���ѧϰС������ͼ��ʾװ�ý�������ʵ�飨������������ˮ���εķ�Ӧ����
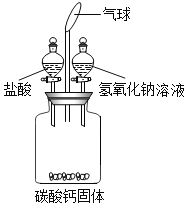
��1��������ƿ�м���һ����������Һ���رջ��������۲쵽����ȫ���ܽ⣬������Ȼ���ٽ�һ��������������Һ������ƿ�У��رջ������������������Ա�С��ͬʱ�ڹ��ƿ�л��ܹ۲쵽��������_____��
��2��ʵ�������ͬѧ�ǶԹ��ƿ�е���Һ����̽����
��������⣩���ƿ�е���Һ������Щ���ʣ�
���������ۣ�С��ȡ���ƿ����Һ���������Թ��ڣ�������ƿ�м������ϡ���ᣬ���������ݲ������ɴ����ó����ۣ����ƿ����Һ���������ΪNa2CO3��NaCl��CaCl2�����һ����ΪС���Ľ����Ǵ���ģ�������_____��������ۣ���ɹ�ʶ���Թ��ƿ��Һ��������ɵ��ж�ֻ�����ֽ��ۣ�����һ��_____�����۶���_____��
��ʵ����ƣ�
ʵ�鲽�� | ʵ������ | ʵ����� |
��ȡ���ƿ����Һ�������Թ��У������м��������_____��Һ | �а�ɫ�������� | ����һ ����ȷ�� |
����������õ���Һ�м�_____ | _____ |