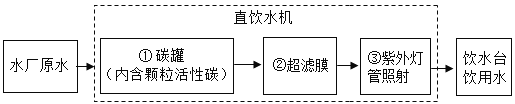
��Ŀ����
����Ŀ��ij��ѧ��ȤС���ͬѧ��ѧϰ���꼶����ѧ���²��е����Ͽ�Ƭ��ʯ�������ʯ���γ���ʱ������������ˮ��̼��Ƶ��������ж�����̼��ˮʱ���ᷴӦ�����ܽ��Խϴ��̼����ƣ�CaCO3+CO2+H2O=Ca��HCO3��2�����뵽ʵ�����г���ʯ��ˮ�������̼��Ӧ������̼��ƣ�Ca(OH)2+CO2=CaCO3��+H2O���Գ�ʱ�������Һ��ͨ��CO2��Ӧ����Һ�е�������ɲ�����Ũ�ȵ���Ȥ��
��������⣩һ����CO2��NaOH��Һ��Ӧ������������ʲô��
���������ϣ���1��ͨ������CO2��Ӧ�Ļ�ѧ����ʽΪ��_____��
��2��ͨ�����CO2����Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+CO2+H2O=2NaHCO3��
��3��̼�����ζ��ǿ�����ˮ�ģ�BaCO3������ˮ��
��4��̼��������Һ�ʼ��ԡ�
��������룩��1������ΪNaOH��Na2CO3��
��2������ΪNa2CO3��
��3������Ϊ_____���ѧʽ����
��4������ΪNaHCO3��
�����ʵ�飩
ʵ�鲽�� | ʵ������ | ʵ����� |
��1���ò�����պȡ��Ӧ����Һ������pH��ֽ�� | pH=9 | ����Һ�Լ��� |
��2��ȡ��Ӧ����Һ�������Թ��У������еμӹ�����BaCl2��Һ | ��_____���� | ���루4�������� |
��3��ȡ���裨2���е��ϲ���Һ������ϡ���� | ������ð�� | ���루1���ͣ�2�������� |
���ó����ۣ����루3��������
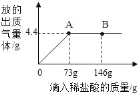
�����۽�������1����ͬѧ�����ʵ�鲽�裨1���Ƕ���ġ�����Ϊ��ʵ������Ƿ���Ҫ��_____��������Ҫ����������Ҫ������
��2��ͬѧ����һ�ΰ�Ŀ��Ͷ���˽̲ģ��������Ȼ�ѹǿ��Сʱ��Ca(HCO3)2=CaCO3��+CO2��+H2O����������ɷ����������NaHCO3���ķ�Ӧԭ����֮���ƣ���д��NaHCO3���ȷֽ�Ļ�ѧ����ʽ_____��
����˼Ӧ�ã�ͬѧ�ǻ�����ʵ���Ҽ��������̼���龰�����룺��������ʯ��ˮ�в���ͨ�������̼���ῴ�������������أ���������һ�£�_____��
���𰸡�CO2+2NaOH�TNa2CO3+H2O NaHCO3��Na2CO3 ��ɫ���� ����Ҫ 2NaHCO3![]() Na2CO3+CO2��+H2O ʯ��ˮ�ȱ���ǣ����ֱ����
Na2CO3+CO2��+H2O ʯ��ˮ�ȱ���ǣ����ֱ����
��������
[��������]��1��ͨ������CO2��Ӧ����̼���ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ��
CO2+2NaOH�TNa2CO3+H2O�����CO2+2NaOH�TNa2CO3+H2O
[�������]
��1������ΪNaOH��Na2CO3����2������ΪNa2CO3����3������ΪNaHCO3��Na2CO3����4������ΪNaHCO3�����NaHCO3��Na2CO3
[���ʵ��]
��1���ò�����պȡ��Ӧ����Һ������pH��ֽ�ϣ�pH=9��˵����Һ�Լ��ԣ�
��2��ȡ��Ӧ����Һ�������Թ��У������еμӹ�����BaCl2��Һ��������ɫ������˵����Һ�к���̼���ƣ�����а�ɫ��������
��3��ȡ���裨2���е��ϲ���Һ������ϡ���ᣬ�������ݣ�˵����Һ�к���̼�����ƣ�ʵ�����������ʾ��
ʵ�鲽�� | ʵ������ | ʵ����� |
��1���ò�����պȡ��Ӧ����Һ������pH��ֽ�� | pH=9 | ����Һ�Լ��� |
��2��ȡ��Ӧ����Һ�������Թ��У������еμӹ�����BaCl2��Һ | �а�ɫ�������� | ���루4�������� |
��3��ȡ���裨2���е��ϲ���Һ������ϡ���� | ������ð�� | ���루1���ͣ�2�������� |
[���۽���]
��1��ʵ�鲽�裨1���Ƕ���ģ�������Ϊ����������Һ��̼������Һ��̼��������Һ�����Լ�����Һ���������Ҫ
��2��NaHCO3���ȷֽ�����̼���ơ�ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪ��2NaHCO3![]() Na2CO3+CO2��+H2O�����2NaHCO3
Na2CO3+CO2��+H2O�����2NaHCO3![]() Na2CO3+CO2��+H2O
Na2CO3+CO2��+H2O
[��˼Ӧ��]
�����ʯ��ˮ�в���ͨ�������̼ʱ���۲쵽ʯ��ˮ�ȱ���ǣ����ֱ���壬������Ϊ��ʼ����̼��Ƴ�������̼��ƺ�ˮ��������̼��Ӧ����������ˮ��̼����ơ����ʯ��ˮ�ȱ���ǣ����ֱ���塣

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�����Ŀ���±��е�Ԫ�ؿ�������������ʡ�
Ԫ������ | �� | ̼ | �� | �� | ͭ |
Ԫ�ط��� | H | C | O | S | Cu |
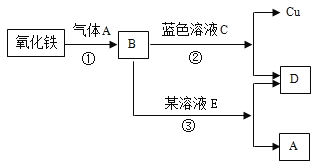
(1)ͭԪ��ͨ����+1��+2�ۣ�ͭԪ������Ԫ�ؿ���ɵ�������______(�û�ѧʽ��ʾ)��
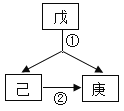
(2)������һ�������·�Ӧ���ɱ��Ͷ�������ʾ��ͼ���£�
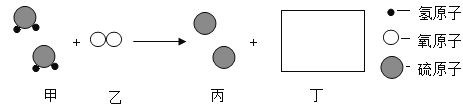
���в�ȫ��Ӧ����ͼʾ________________��
�ڴ��۽ǶȽ��ͻ�ѧ�仯ǰ�������غ��ԭ���� ______��
(3)�졢�������dz��г������ʣ�Ҳ�����ϱ��е�һ�ֻ�����Ԫ����ɵģ���������ͼ��ʾ��ת����ϵ�����졢�������Ԫ����ͬ����д�����Ļ�ѧʽ�� ________��