��Ŀ����
����Ŀ��ijѧϰС��ͬѧ���ø��������ʵ��ʱ����մ�����ϣ�����Ƥ��������غ�ɫ����ʦ�ṩ������Һ��ͬѧϴ�֣��غ�ɫ������ʧ��ͬѧ�Բ����������Ȥ���������ʦ��֪������(H2C2O4)��ͨ��״����Ϊ���壬��һ��������ˮ���ᣬ�㷺������ֲ��ԴʳƷ�С����������Ũ����Ĵ������£����ȷֽ�����̼���������ˮ��С��ͬѧ����������̼����������������������̽����
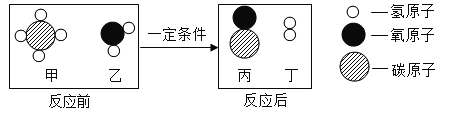
��������⣩�������ȷֽ�������������ļ���̼�������
�����в��룩����1��ֻ��CO ����2��ֻ��CO2 ����3��ͬʱ����CO��CO2��
���������ϣ�
��һ����̼���������ʯ��ˮ��Ӧ��
��Ũ��������ˮ�ԣ������������л��е�ˮ������ʹ�������
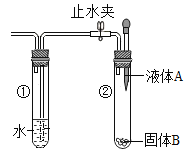
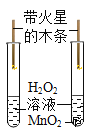
��ʵ��̽����ͬѧ�������ͼ1��ʾװ�ý���ʵ�飺
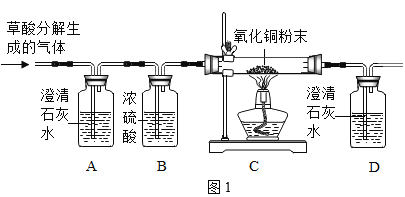
(1)ʵ��ʱ�����۲쵽װ��A�е�����Ϊ___��˵������ķֽ��������CO2������ڡ�
(2)Ϊ��ʵ�鰲ȫ����ʵ�鿪ʼӦ��ͨһ������壬��___��ʵ������У��۲쵽C��������___���ɴ˵ó�����ֽ������CO������ڡ�ͬѧ����Ϊ������װ�ô������Ե�ȱ�ݣ���ĸĽ�������___��
(3)װ��C��ֱ�������ڷ�����Ӧ�Ļ�ѧ����ʽΪ___��
��ʵ����ۣ�(4)ͨ��ʵ�飬�ɵõ��Ľ����Dz���3��ȷ����д��������Ũ��Ĵ��£����ȷֽ�Ļ�ѧ����ʽ��___��
���������ۣ�(5)�����������ط�Ӧ�Ļ�ѧ����ʽΪ��3H2C2O4+2KMnO4��K2CO3+2MnO2+3H2O+5CO2������֪�ڻ�ѧ��Ӧ�У���Ԫ�ػ��ϼ����ߵķ�Ӧ��Ϊ��ԭ������Ԫ�ػ��ϼ۽��͵ķ�Ӧ��Ϊ����������˷�Ӧ�е���������___��
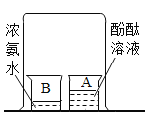
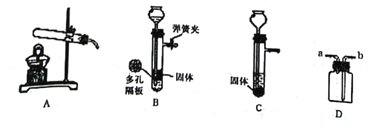
���������㣩(6)�ⶨ���������Ʒ�и�����ص���������(���������Ʒ�к���������)��ͬѧ���������ͼ2��ʵ��װ�ã�ȡ1g������ع�����Ʒ�����Թ��У�ʵ���н�ע�����ڵIJ�����Һ(����)��ȫע���Թ��С����Թ��в��ٲ�������ʱ�����õ���Ͳ��ˮ�����Ϊ330��������֪ʵ�������¶�����̼���ܶ�Ϊ2g/L���������Ʒ�и�����ص���������___��(д���������)
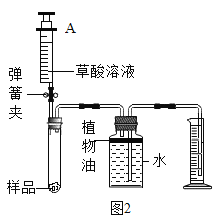
��ʵ�鷴˼��(7)ͼ2��ˮ���Ϸ�����һ��ֲ���ͣ�Ŀ����___��ʵ�������ѧϰС���ͬѧ�Ը�ʵ������˷�˼��ͬѧ����Ϊ�ø�װ�ò�õ���Ʒ�и�����ص���������ƫ�ߣ�����Ϊԭ����___��
���𰸡�����ʯ��ˮ����� ���� ��ɫ�Ĺ����ɺ�ɫ ��β���ĵ��ܿڷ�һ����ȼ�ľƾ��ƻ��һ������(��������) CO+CuO![]() Cu+CO2 H2C2O4
Cu+CO2 H2C2O4 CO2+CO+H2O �������(��KMnO4) ���ݴ�װ�ÿ�֪����Ͳ�ڵ�ˮ�����Ϊ������������������˲����Ķ�����̼�����Ϊ330mL��ʵ�������¶�����̼���ܶ�Ϊ2g/L���������̼������Ϊ��2g/L��0.33L=0.66g��
CO2+CO+H2O �������(��KMnO4) ���ݴ�װ�ÿ�֪����Ͳ�ڵ�ˮ�����Ϊ������������������˲����Ķ�����̼�����Ϊ330mL��ʵ�������¶�����̼���ܶ�Ϊ2g/L���������̼������Ϊ��2g/L��0.33L=0.66g��
�������ص�����Ϊx��
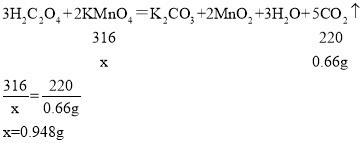
�ʸ�����ص���������Ϊ��![]() �� ��ֹ������̼����ˮ ����IJ�����Һռ�����Թ��ڵIJ��������ʹ��õĶ�����̼�����ƫ��
�� ��ֹ������̼����ˮ ����IJ�����Һռ�����Թ��ڵIJ��������ʹ��õĶ�����̼�����ƫ��
��������
��1�������Dz���ķֽ��������CO2������ڣ���װ��A����ʯ��ˮ�л�۲쵽ʯ��ˮ����ǡ�
��2������ֽ����ɵ������п��ܺ���CO������Ҫ�Ƚ�װ���ڵĿ�����ȫ�ž��ٵ�ȼ�ƾ��ƣ���ֹCO�������Ϸ�����ը�������ɵ���������CO����CO��������ͭ��ĩ������Ӧ�����Cװ�ÿɹ۲쵽��ɫ�Ĺ�������һ����ͨ���۲�����ʵ��װ�÷��ֲ���β������װ�ã���CO�ж��ԣ���˲����ŷŵ������У���Ҫ�ռ���ֱ�ӵ�ȼ����˿���Dװ�ú����һյ��ȼ�þƾ��ƻ���һ�������ռ�CO��
��3��װ��C������ͭ����ԭ����ѧ����ʽΪ��CO+CuO![]() Cu+CO2��
Cu+CO2��
��4������3�ǼȲ���CO����CO2�������ֽ�û�ѧ����ʽΪ��H2C2O4 CO2+CO+H2O��
CO2+CO+H2O��
��5�������У�HԪ�ػ��ϼ�Ϊ+1�ۣ�OԪ�ػ��ϼ�Ϊ-2�ۣ����ݻ�ѧʽ�л��ϼ۴�����Ϊ0��֪CԪ�صĻ��ϼ�Ϊ+3�ۡ���������У�KΪ+1�ۣ�OΪ-2�ۣ����MnԪ�صĻ��ϼ�Ϊ+7�ۡ���Ӧ��CԪ�ػ��ϼ�������+4�ۣ�MnԪ�ػ��ϼ۽��͵�+4�ۣ����������ΪKMnO4��
��6�����ݴ�װ�ÿ�֪����Ͳ�ڵ�ˮ�����Ϊ������������������˲����Ķ�����̼�����Ϊ330mL��ʵ�������¶�����̼���ܶ�Ϊ2g/L���������̼������Ϊ��2g/L��0.33L=0.66g��
�������ص�����Ϊx��
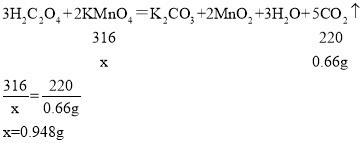
�ʸ�����ص���������Ϊ��![]() ��
��
��7�������Ķ�����̼������ˮ�������ֲ���ͽ�������ˮ��������ֹ������̼����ˮ����ʵ���н�ע����A�еIJ�����Һ��ѹ�����Թ��ڣ��ᵼ���Թ�������ѹǿ�������ʵ�������Ϊ������IJ�����Һռ�����Թ��ڵIJ��������ʹ��õĶ�����̼�����ƫ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�