��Ŀ����
����Ŀ��ˮ������֮Դ����ش��й�ˮ���������⣺
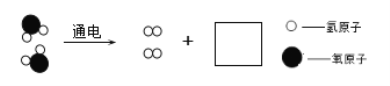
��1�����ˮʵ����ͼ��ʾ��
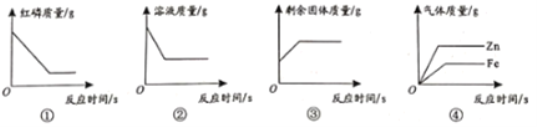
���������������������ԼΪ_____���÷�Ӧ����ʾ��ͼ���£����ڷ����ڲ�ȫ��Ӧ����ʾ��ͼ_____��
��2�����������ܡ���һ�ֱ�Яʽ���⾻ˮ�����侻ˮԭ������ͼ��
![]()
�ٹ�������ȥ��_____��������ԡ��������ԡ������ʡ�
�ڿ������Ը����������л���̿�����ӽ�����֬�ȡ�����̿�������_____���á����ӽ�����֬��ȥ��ԭˮ�еĸ����ӣ������ӵķ�����_____��
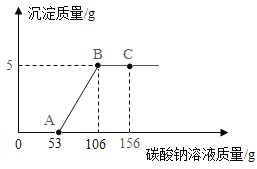
��3����ͼ��A��B��C���ֹ������ʵ��ܽ������ͼ������ͼʾ�ش��������⣺
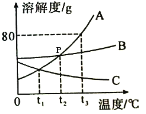
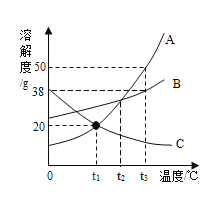
��p������_____��
�������£���ʢ��A�ı�����Һ���Թܷ���ʢˮ���ձ��У������ձ��ڵ�ˮ�м���һ�����IJ�NH4NO3�����裬�Թ��ڿɹ۲쵽��������_____��
�۽�t3��ʱA��B��C�������ʵı�����Һ���µ�t1�棬������Һ�����ʵ������������ɴ�С�Ĺ�ϵ��_____��
���𰸡�1��2 ![]() ������ ���� Ca2+ t2��ʱA��B���ܽ����� �й������� B>A>C
������ ���� Ca2+ t2��ʱA��B���ܽ����� �й������� B>A>C
��������
���ݵ��ˮ��������۱��ʣ�ˮ�ľ������ܽ�����ߵ�Ӧ�ú���������������֪ʶ���з�������
��1����ͼ֪������������ұ�������������������������������ԼΪ1��2�����������غ㶨�ɻ�ѧ��Ӧǰ��ԭ�ӵ���Ŀ������䣬�����ڲ�ȫ��Ӧ����ʾ��ͼ��![]() ��
��
��2���ٹ�������ȥ�����������ʣ�
�ڻ���̿��������������ã������ӵķ�����Ca2+��
��3����p��ĺ�����t2��ʱA��B���ܽ����ȣ�
�������£���ʢ��A�ı�����Һ���Թܷ���ʢˮ���ձ��У������ձ��ڵ�ˮ�м���һ�����IJ�NH4NO3�����裬NH4NO3����ˮ���ȣ�ʹ��Һ���¶Ƚ��ͣ�A���ܽ�����¶Ƚ��Ͷ����ͣ��Թ��ڻ���A�Ĺ���������
�۽�t3��ʱA��B��C�������ʵı�����Һ���µ�t1����A��B���ܽ�����¶Ƚ��Ͷ����ͣ�t1�� B���ܽ�ȴ���A��C��t3��ʱ�ı�����Һ���t1���IJ�������Һ�������е������������䣬������t1��ʱ������Һ�����ʵ������������ɴ�С�Ĺ�ϵ��B>A>C��

 С�����ϵ�д�
С�����ϵ�д�����Ŀ�������й����ʵļ��顢���֡����롢�ᴿ���õ��Լ����������
ѡ�� | ʵ��Ŀ�� | �����Լ��� |
A | ���� | NaOH��Һ�� |
B | ��ȥ��ʯ���к�������ʯ��ʯ | ˮ��ϡ���� |
C | �� | ���˻����� |
D | ����ʧȥ��ǩ��Ũ�����ϡ���� | ˮ��Сľ�� |
A. A B. B C. C D. D
����Ŀ��ijУ��ѧ��ȤС��Ϊ�ⶨ��ͭ��п��������������������ʵ�飺
ʵ�鲽�� | �ٳ�ȡ�ձ������� | �ڽ�������������ձ��в����� | �۳�ȡ��ͭ��Ʒ�����ձ��У�ʹ֮������ǡ����ȫ��Ӧ | ���ط�Ӧ��ȫ���� |
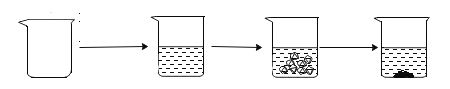
ʵ��ͼʾ |
| |||
ʵ������ | �ձ�������Ϊ50.0g | �ձ������������Ϊ150.0g | ��ͭ��Ʒ������Ϊ20.0g | �ձ������л�������Ϊ169.6g |
�漰��Ӧ�Ļ�ѧ����ʽ��Zn+2HCl==ZnCl2+H2��������������⣺
��1������ۿɹ۲쵽�������ǣ�_____��
��2����ʵ�������ɵ�������������_____g��
��3����û�ͭ��Ʒ��п������������
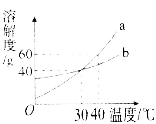
����Ŀ���ҹ���ѧ�Һ�°����ĺ����ƼΪ���ҵ������չ�����ܳ����ס����Ʊ�����������漰Na2CO3��NaCl��NH4Cl��NaHCO3�����ʡ������±����ݻش�:
�¶�/��C | 0 | 10 | 20 | 30 | 40 | 50 | 60 | |
�ܽ��/g | Na2CO3 | 7 | 12.2 | 21.8 | 39.7 | 48.8 | 47.3 | 46.4 |
NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 | |
NH4Cl | 29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 55.2 | |
NaHCO3 | 6.9 | 8.2 | 9.6 | 11.1 | 12.7 | 14.5 | 16.4 | |
��1����50��Cʱ����100gˮ�м���48gNa2CO3��ֽ���������Һ����Ϊ______�������ձ��и������������䣬������40��Cʱ��������Һ���ʵ�����������____(������С���������������������)��
��2�����ݱ����������ʵ��ܽ�ȣ��ش��������⣺
��60��Cʱ���ֱ�������������ֹ��������м�ˮ��ɱ�����Һ��������Һ����������__��
�ں����Ƽ��ԭ���ǣ�NaCl+NH3+H2O+CO2=NH4Cl+NaHCO3����������̼�����ƺ��Ȼ���У�����������_______��������_______��