��Ŀ����
Ӳ���������Ͻ���Ϊ�����ܶȽ�С��ǿ�Ƚϴ���ʴ�Խ�ǿ���ص㣬����������ɻ�������ɴ���������ϡ���֪Ӳ����ĩ�����⣬������þ��ͭ�е�һ�ֻ����֡�ij��ѧ��ȤС������ʦ��ָ���£���Ӳ����ĩ��þ��ͭ�Ĵ������������̽����
�������ϣ���������������Һ��Ӧ����ʽΪ2Al+2NaOH+2H2O��2NaAlO2+3H2��������NaAlO2����ˮ����Mg��Cu��������������Һ��Ӧ��
��������룩
����1���úϽ��ĩ�г�Al�⣬������Mg��
����2���úϽ��ĩ�г�Al�⣬������Cu��
����3���úϽ��ĩ�г�Al�⣬������_____���ѧʽ����
��ʵ��̽��������ʵ�����ѡ����Լ���10%���ᡢ30%NaOH��Һ��
ʵ�鷽�� | ʵ������ | ���� |
��ȡһ������Ӳ����ĩ���ӹ�����_____����ַ�Ӧ����ˣ��������á� | ��ĩ�����ܽ⣬��������ų��� | Ӳ����һ������_____�� |
��ȡ����������������ӹ�����_____����ַ�Ӧ�� | ���������ܽ⣬��������ų��� | Ӳ����һ������_____�� |
���ó����ۣ�������_____������
�����⣺����ȷ�ش�����С�⣬�����4�ֵĽ���������ѧ�Ծ��ֲܷ�����60�֡�
����չ������������������ͬ��ʵ�鷽������֤����ͭ�Ľ������ǿ����
����һ��_____��
��������_____��
���й����ܽ���ȫ��ȷ��һ���ǣ�������
A��ѧ����ᷢչ | B��ѧ�뽡�� |
�ٹ㷺ʹ��̫��������Ч����̼�ŷ� �ڲ��������ڸ��ϲ��� ��úȼ�ղ����Ķ����������γ����� | ��ȱ�������ƶѪ ����������������Ҫ�Ĺ������� ���ؽ������ж�������ţ�̡��������� |
C��ѧ�밲ȫ | D��ѧʵ���е������ |
�ٽ����ܶ�ǰ���ƻ�ʵ�� ��ú��й¶�������Ʋ���й¶ ��Ƥ��մ��Ũ���ᣬ������������Һ�к� | ����ȡ���壺�ȼ�ҩƷ����װ�������� �ڳ���һ������ҩƷ���ȼ�ҩƷ������� �ۿ�ȼ������ȼ�գ����鴿���ȼ |
A. A B. B C. C D. D





 ������ƿ B.
������ƿ B.  ��ͭ��
��ͭ�� ����� D.
����� D.  ���������
���������


 B.
B.  C.
C.  D.
D. 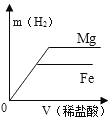
 ��ȼ�ƾ��� B.
��ȼ�ƾ��� B.  ��������ζ
��������ζ ������Ƥ�� D.
������Ƥ�� D.  ��Ͳ����
��Ͳ����