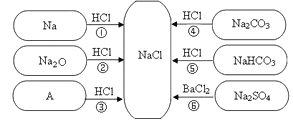
��Ŀ����
����Ŀ��ʯ��ʯ�������������Ȼ��Ƶȶ��ֻ�����Ʒ��ԭ�ϡ�ij�о���ѧϰС��Ϊ�˲ⶨ���ؿ�ɽʯ��ʯ��̼��Ƶ�����������ȡ���˿�ʯ��Ʒ����ȡϡ����200g��ƽ���ֳ�4�ݣ�����ʵ�飬������£�
ʵ �� | 1 | 2 | 3 | 4 |
������Ʒ����/g | 5 | 10 | 15 | 20 |
����CO2������/g | 1.76 | 3.52 | 4.4 | m |
(1)�ڼ��η�Ӧ�п�ʯ��ʣ�ࣿ_________��
(2)����mֵ��________��
(3)�Լ�������ʯ��ʯ��̼��Ƶ���������Ϊ_________��
���𰸡� �� �� ��
�������������������1�����ݱ�����ǰ���ε����ݷ�����֪��ÿ����5g��Ʒ������CO2������Ϊ1.76g���ʵ�3�μ���15g��Ʒ��Ӧ������CO2������Ϊ1.76g��3=5.28g����ʵ��ֻ��4.4g��˵����ʱ��������ȫ��Ӧ���ʵ�3��4��ʵ����̼�����ʣ�ࡣ
��2����Ϊ��3����������ȫ��Ӧ������������������ʵ�4�η�Ӧ���ɵ����������͵�3�ε�һ���࣬��m=4.4��
��3�����ݵ�1�λ��2��ʵ���ж�����̼����������ϻ�ѧ����ʽ�ж�����̼��̼��Ƶ������ȣ���������μӷ�Ӧ��CaCO3���������ٸ�������������ʽ���м��㼴�ɡ�
�⣬���1��ʵ������Ʒ�е�̼��Ƶ�����Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 1.76g
100��44 =x��1.76g
��ã�x=4g
���ԣ�ʯ��ʯ��̼��Ƶ���������Ϊ![]() ��100%=80%
��100%=80%
��ʯ��ʯ��̼��Ƶ���������Ϊ80%��