��Ŀ����
����Ŀ����ͼ��ʵ������ȡ����ij���װ�裬��ش��������⣺
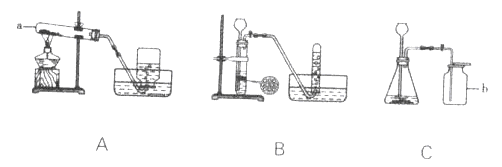
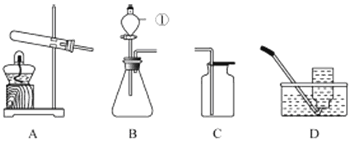
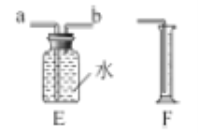
��1��д��ͼ�б�����������ƣ�a______��b______��
��2��ʵ���ҿɼ���KMnO4������ȡO2 ��
����д���÷�Ӧ�Ļ�ѧ����ʽ______��
����A��ʾ������һ��ʱ���ijͬѧ�����Թܿ�δ�������ţ�Ӧ��ȡ����ȷ������______������װ����������ʵ�顣
��3��ʵ���ҿ���п����ϡ���ᷴӦ��ȡH2����д���÷�Ӧ�Ļ�ѧ����ʽ��______��Ӧѡ�õ�װ����______������ĸ����
��4��ʵ����ѡ��Cװ����ȡCO2�Ļ�ѧ����ʽ��CaCO3+2HCl=CaCl2+H2O+CO2��
����㣺
��CO2����Է���������______��
�������ռ�4 ƿ��ÿƿ0.125L) CO2���壨ʵ��������CO2���ܶ�Ϊ2g�� L-1������������____g��
���ռ�����CO2������Ҫ����ʯ����CaCO38O%����������______g ����ȷ��0.1g����
���𰸡���1���Թܼ���ƿ����2����2KMnO4![]() K2MnO4+MnO2+ O2��
K2MnO4+MnO2+ O2��
�� �������Ƴ�ˮ�棬Ϩ�����ƣ���װ����ȴ�ο����������Թܿڷ������ţ����������𰸾��ɣ�
��3��Zn+H2SO4=ZnSO4+ H2��, B
��4����44 �� 1 �� 2.8 ����2.9�����������𰸾��ɣ�
����������1���Թܼ���ƿ����2������������ڼ��������·ֽ���������ء��������̡���������Ӧ�Ļ�ѧ����ʽ2KMnO4![]() K2MnO4+MnO2+ O2������ �������Ƴ�ˮ�棬Ϩ�����ƣ���֮����ˮ�������Թ��ڣ�ʹ�Թ�ը�ѣ���װ����ȴ�ο����������Թܿڷ������ţ���ֹ������ؿ����������ܡ���3��п����ϡ���ᷴӦ��������п��H2����Ӧ�Ļ�ѧ����ʽ��Zn+H2SO4=ZnSO4+ H2��, ѡ����װ���迼���������ǣ���Ӧ���״̬�ͷ�Ӧ���������ȹ�����ȡ���壬����װ��ΪA�������Һ�峣���·�Ӧ��ȡ����Ӧѡ�õķ���װ��ΪBC��ѡ���ռ�װ���迼��������ܶȣ��Ƿ�������ijɷַ�Ӧ��������ˮ�е��ܽ��ԡ��������ܶȱȿ�����С�������������ſ������ռ�������������ˮ��������ˮ���ռ���Ӧѡ�õ�װ����B����4���٢���Է�������=�����ԭ��������ԭ�Ӹ�����֮�ͣ�CO2����Է���������44 ��4ƿ��ÿƿ0.125L) CO2���壨ʵ��������CO2���ܶ�Ϊ2g�� L-1������������0.125L��2g L-1��4=1g�� ����ȡ1g������̼��Ҫ̼��Ƶ�����Ϊx��CaCO3+2HCl=CaCl2+H2O+CO2��
K2MnO4+MnO2+ O2������ �������Ƴ�ˮ�棬Ϩ�����ƣ���֮����ˮ�������Թ��ڣ�ʹ�Թ�ը�ѣ���װ����ȴ�ο����������Թܿڷ������ţ���ֹ������ؿ����������ܡ���3��п����ϡ���ᷴӦ��������п��H2����Ӧ�Ļ�ѧ����ʽ��Zn+H2SO4=ZnSO4+ H2��, ѡ����װ���迼���������ǣ���Ӧ���״̬�ͷ�Ӧ���������ȹ�����ȡ���壬����װ��ΪA�������Һ�峣���·�Ӧ��ȡ����Ӧѡ�õķ���װ��ΪBC��ѡ���ռ�װ���迼��������ܶȣ��Ƿ�������ijɷַ�Ӧ��������ˮ�е��ܽ��ԡ��������ܶȱȿ�����С�������������ſ������ռ�������������ˮ��������ˮ���ռ���Ӧѡ�õ�װ����B����4���٢���Է�������=�����ԭ��������ԭ�Ӹ�����֮�ͣ�CO2����Է���������44 ��4ƿ��ÿƿ0.125L) CO2���壨ʵ��������CO2���ܶ�Ϊ2g�� L-1������������0.125L��2g L-1��4=1g�� ����ȡ1g������̼��Ҫ̼��Ƶ�����Ϊx��CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
X 1g
100/x=44/1g x=![]() ������Ҫ����ʯ����CaCO38O%����������:
������Ҫ����ʯ����CaCO38O%����������: ![]() ��80%��2.84g
��80%��2.84g

 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д�