��Ŀ����
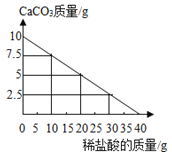
����Ŀ����ѧʵ��С��ͬѧΪ�о�ij�����������ƻ�����ʴ�ij̶ȣ��� 6.4g ���������Ƭ������һŨ�ȵ�ϡ�����г�ַ�Ӧ����ò���������������ϡ�����������ϵ����ͼ ��ʾ��������Ƭ���� Fe2O3 �⣬�����������ʣ���
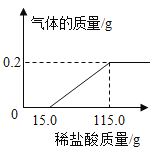
��1����������������Ϊ_____g��
��2����ϡ���������ʵ���������_______�������Ƭ�� Fe2O3 ����������Ϊ����_________����д��������̣������ȷ����0.1%��
���𰸡�0.2g 7.3% 12.5%
��������
![]()
��������ϡ���ᷴӦ�����Ȼ�����ˮ������ϡ���ᷴӦ�����Ȼ�������������
��1����ͼ��֪����������������Ϊ0.2g��
��2����ϡ���������ʵ���������Ϊx
������Ӧ��ϡ���������Ϊ![]()
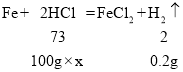
![]()
![]()
����Ƭ�� Fe2O3 ������Ϊy
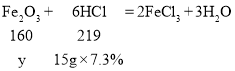
![]()
![]()
����Ƭ�� Fe2O3 ����������Ϊ![]()
��ϡ���������ʵ���������Ϊ7.3%����Ƭ�� Fe2O3 ����������Ϊ12.5%��

����Ŀ����Դ��ʳƷ����������������ȫ���ȵ㡣���й۵��������ȫ��ȷ���ǣ� ��
A.�������� | B.����ˮ��Դ |
a.��ȼ�̻�����-���� b. | a.������ˮ����-��Լ��ˮ b.����̿��ˮ-���������� |
C.ʳƷ���� | D.��Դ���� |
a.���ʺ���Ʒ-�ü�ȩ��Һ���� b.�ӳ�ʳƷ������-���Ӵ����ķ����� | a.���ִ�½��ȼ��-������ԴΣ�� b.�ƹ㳵���Ҵ�����-��β����Ⱦ |
A.AB.BC.CD.D