��Ŀ����
��9�֣�ʵ����ѧϰ��ѧ����Ҫ�ֶΣ�ͨ��ʵ���������ʵ�������ʹ��¾���
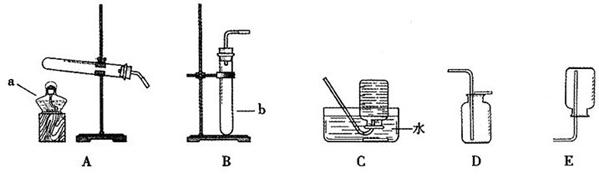
��һ��ʵ������ȡ�����dz���һ����Ҫ��ʵ�飬�������������װ��ͼ�ش��й����⣺
װ��I װ�â� װ�â�
��1��װ��I���ø����������������Ӧ�Ļ�ѧ����ʽΪ_________________________�����������Ѿ�������ʵ�������� ��
��2��ijͬѧ��װ�â���ȡ���ռ�������̼����ȼ�ŵ�ľ�����ڼ���ƿ��������ʼ��δ���ֻ���Ϩ��ԭ����______________________________________________��
��3����װ�â���ж������̺�˫��ˮ��ȡ������ʵ��ʱ������Ӧ���ʹ��죬���Բ�ȡ�Ĵ��������� ��
��4�������Ѿ�ѧ��ʵ������ȡO2��CO2��H2��������ķ�Ӧԭ������ȡ���ռ�������������ɳ���ȡ����������ʱ��Ӧ�Ĺ�ͬ��_______����ѡ����ţ���
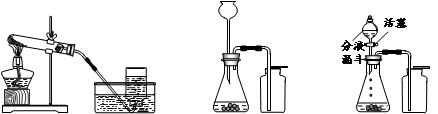
������ѡ������װ�òⶨˮ��Ԫ����ɣ���ش����⣺
��5��Ϊȷ�ⶨˮ����ɲ���ֹ���ʡ������ȸ��ţ����ܿڵ���ȷ����˳��Ϊ��
�ۡ�___��____��____��____���١��ڡ��������⡣
��6�������Aװ������Ʒ��ʵ��������6.4�ˣ�Eװ������������7.2�ˣ�Fװ��������0.1�ˣ��ݴ˿����ˮ��H��OԪ��������Ϊ��ֻд����ʽ��____________��
��7��ʵ���������A�����к�ɫ���壬���ʵ������Ӱ����________________�����������ƫ�����������ƫ������Ӱ�족����
��һ��ʵ������ȡ�����dz���һ����Ҫ��ʵ�飬�������������װ��ͼ�ش��й����⣺

װ��I װ�â� װ�â�
��1��װ��I���ø����������������Ӧ�Ļ�ѧ����ʽΪ_________________________�����������Ѿ�������ʵ�������� ��
��2��ijͬѧ��װ�â���ȡ���ռ�������̼����ȼ�ŵ�ľ�����ڼ���ƿ��������ʼ��δ���ֻ���Ϩ��ԭ����______________________________________________��
��3����װ�â���ж������̺�˫��ˮ��ȡ������ʵ��ʱ������Ӧ���ʹ��죬���Բ�ȡ�Ĵ��������� ��
��4�������Ѿ�ѧ��ʵ������ȡO2��CO2��H2��������ķ�Ӧԭ������ȡ���ռ�������������ɳ���ȡ����������ʱ��Ӧ�Ĺ�ͬ��_______����ѡ����ţ���
| A����Ҫ���� | B����ʹ�ô��� |
| C��û������μӷ�Ӧ | D�����ɵ�����ֻ��һ�� |

��5��Ϊȷ�ⶨˮ����ɲ���ֹ���ʡ������ȸ��ţ����ܿڵ���ȷ����˳��Ϊ��
�ۡ�___��____��____��____���١��ڡ��������⡣
��6�������Aװ������Ʒ��ʵ��������6.4�ˣ�Eװ������������7.2�ˣ�Fװ��������0.1�ˣ��ݴ˿����ˮ��H��OԪ��������Ϊ��ֻд����ʽ��____________��
��7��ʵ���������A�����к�ɫ���壬���ʵ������Ӱ����________________�����������ƫ�����������ƫ������Ӱ�족����
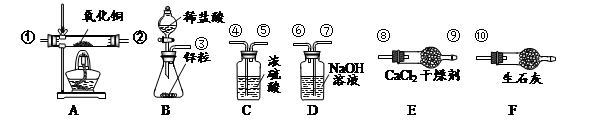
��1��2KMnO4 K2MnO4+MnO2+O2����ƿ��������ð����
��2������©��û������Һ���£���©��û��Һ�⣬��װ��©���������Բ��ò����֣���
��3����ת��Һ©��������������Һ���µ����ʡ���4��CD����5���ߢޢܢݣ�ȫ�ԲŸ��֣���
��6��(7.2��6.4):6.4�������������Ҳ���֣���ע�⣺��0.1�أ�����7����Ӱ�졣
��2������©��û������Һ���£���©��û��Һ�⣬��װ��©���������Բ��ò����֣���
��3����ת��Һ©��������������Һ���µ����ʡ���4��CD����5���ߢޢܢݣ�ȫ�ԲŸ��֣���
��6��(7.2��6.4):6.4�������������Ҳ���֣���ע�⣺��0.1�أ�����7����Ӱ�졣
��һ����1��������ؼ�����������ء��������̺���������Ӧ�Ļ�ѧ����ʽ2KMnO4 K2MnO4+MnO2+O2���������Ѿ�������ʵ��������ƿ��������ð����
K2MnO4+MnO2+O2���������Ѿ�������ʵ��������ƿ��������ð����
��2��IIװ���г���©��û������Һ�棬����ijͬѧ��װ�â���ȡ���ռ�������̼����ȼ�ŵ�ľ�����ڼ���ƿ��������ʼ��δ���ֻ���Ϩ��
��3������˫��ˮ�����������Ʒ�Ӧ���ʣ�����Ӧ���ʹ��죬���Բ�ȡ�Ĵ�����������ת��Һ©��������������Һ���µ����ʣ�
��4��O2��CO2��H2��������ķ�Ӧԭ���Լ�������������ʿ�֪����������ʱ��Ӧ�Ĺ�ͬ��û������μӷ�Ӧ�����ɵ�����ֻ��һ�֣�
��������5��ʵ��������п��ϡ������ȡ����ʱ���������ӷ��������Ƶõ������ڻ�������HCl�����ˮ���������õ������������H2���������HCl�����ˮ���������ܿڵ���ȷ����˳��Ϊ���ۡ��ޡ��ܡ��ݡ��١��ڡ��������⣻
��6�����÷�Ӧǰ���С��������ͭ�е�����Eװ�����������Ƿ�Ӧ����ˮ��������֪����Ԫ�ص�����Ϊ6.4g��ˮ������Ϊ7.2g������ˮ��H��OԪ��������Ϊ��7.2-6.4����6.4��
��7������ͨ����װ�õı仯���ⶨ�⡢��Ԫ�ص��������������к�ɫ���壬˵������ʣ�࣬������Ԫ������Ԫ���ǰ�һ����������ˮ�ģ����ʵ������Ӱ������Ӱ�죮
 K2MnO4+MnO2+O2���������Ѿ�������ʵ��������ƿ��������ð����
K2MnO4+MnO2+O2���������Ѿ�������ʵ��������ƿ��������ð������2��IIװ���г���©��û������Һ�棬����ijͬѧ��װ�â���ȡ���ռ�������̼����ȼ�ŵ�ľ�����ڼ���ƿ��������ʼ��δ���ֻ���Ϩ��
��3������˫��ˮ�����������Ʒ�Ӧ���ʣ�����Ӧ���ʹ��죬���Բ�ȡ�Ĵ�����������ת��Һ©��������������Һ���µ����ʣ�
��4��O2��CO2��H2��������ķ�Ӧԭ���Լ�������������ʿ�֪����������ʱ��Ӧ�Ĺ�ͬ��û������μӷ�Ӧ�����ɵ�����ֻ��һ�֣�
��������5��ʵ��������п��ϡ������ȡ����ʱ���������ӷ��������Ƶõ������ڻ�������HCl�����ˮ���������õ������������H2���������HCl�����ˮ���������ܿڵ���ȷ����˳��Ϊ���ۡ��ޡ��ܡ��ݡ��١��ڡ��������⣻
��6�����÷�Ӧǰ���С��������ͭ�е�����Eװ�����������Ƿ�Ӧ����ˮ��������֪����Ԫ�ص�����Ϊ6.4g��ˮ������Ϊ7.2g������ˮ��H��OԪ��������Ϊ��7.2-6.4����6.4��
��7������ͨ����װ�õı仯���ⶨ�⡢��Ԫ�ص��������������к�ɫ���壬˵������ʣ�࣬������Ԫ������Ԫ���ǰ�һ����������ˮ�ģ����ʵ������Ӱ������Ӱ�죮

��ϰ��ϵ�д�
�����Ŀ