��Ŀ����
����Ŀ���������ƣ�Na2O2����һ�ֵ���ɫ��ĩ������ΪDZˮͧ�Ĺ��������˺����������Ҫ�ɷ��е�����������������̼��ˮ������������̼��ˮ�ֱܷ���Na2O2��Ӧ����������Ϊ��̽��Na2O2��DZˮͧ�з�Ӧ������ʣ�С��ͬѧ�������ͼ1��ʾ��ģ��ʵ�顣��ش�
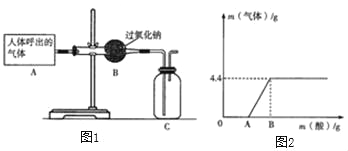
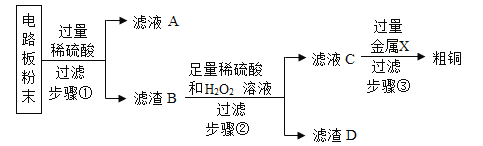
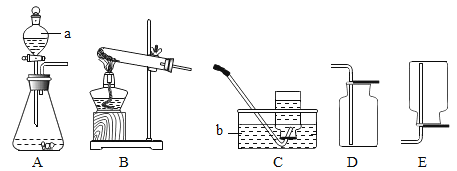
��1������C���Ƿ�ʢ�������ķ�����_____��
��2��ȡ������Ӧ�������еĹ�����Ʒ���Ƴ���Һû�����������
��ȡһ������2������Һ�ȵμӹ�����BaCl2��Һ���������������а�ɫ�������ɣ���ط�Ӧ�Ļ�ѧ����ʽΪ_____�����ˡ������Һ��pH��7������������_____������Һ�еμ���ɫ��̪��Һ����Һ��Ϊ_____ɫ��
��д���������Ʒֱ���ˮ�Ͷ�����̼��Ӧ�Ļ�ѧ����ʽ_____��
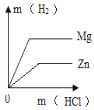
��3����ȡһ������2������Һ��������εμ�ϡ���������������������ϡ����������Ĺ�ϵ��ͼ2��ʾ��
������Ϳ�ʼ�μ�ϡ����ʱû�в��������ԭ��_____��
���������Ʒ��̼���Ƶ�����_____��
���𰸡���Cװ�õĵ��ܿڴ���һ�������ǵ�ľ������ľ����ȼ��֤���ռ����� Na2CO3+BaCl2��BaCO3��+2NaCl ȡ��Һ���� �� 2Na2O2+2H2O�T4NaOH+O2����2Na2O2+2CO2��2Na2CO3+O2 ���������ƺ�̼���ƵĻ��Һ�м���ϡ���ᣬϡ���������������Ʒ�Ӧ 10.6g
��������
��1����������������ȼ�ԣ����Լ���C���Ƿ�ʢ�������ķ����ǣ���Cװ�õĵ��ܿڴ���һ�������ǵ�ľ������ľ����ȼ��֤���ռ����ˡ������Cװ�õĵ��ܿڴ���һ�������ǵ�ľ������ľ����ȼ��֤���ռ����ˣ�
��2����ȡһ������2������Һ�ȵμӹ�����BaCl2��Һ���������������а�ɫ�������ɣ��ó�����̼�������Ȼ�����Ӧ���ɵ�̼�ᱵ��������ط�Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+BaCl2��BaCO3��+2NaCl�����ˡ������Һ��pH��7�����������ǣ�ȡ��Һ����������Һ�еμ���ɫ��̪��Һ����Һ��Ϊ��ɫ��˵������Һ�к����������ơ������Na2CO3+BaCl2��BaCO3��+2NaCl��ȡ��Һ�������죻
���������Ʒֱ���ˮ�Ͷ�����̼��Ӧ�Ļ�ѧ����ʽ�ֱ��ǣ�2Na2O2+2H2O�T4NaOH+O2����2Na2O2+2CO2��2Na2CO3+O2�����2Na2O2+2H2O�T4NaOH+O2����
2Na2O2+2CO2��2Na2CO3+O2��
��3������ʼ�μ�ϡ����ʱû�в��������ԭ���ǣ����������ƺ�̼���ƵĻ��Һ�м���ϡ���ᣬϡ���������������Ʒ�Ӧ��
������Ʒ��̼���Ƶ�����Ϊx��
Na2CO3+2HCl��2NaCl+H2O+CO2��
![]()
![]() ��ã�x��10.6g
��ã�x��10.6g
��������������ƺ�̼���ƵĻ��Һ�м���ϡ���ᣬϡ���������������Ʒ�Ӧ��������Ʒ��̼���Ƶ�������10.6g��

����Ŀ��ijУѧϰС����̽������IJⶨ�����ݴ���������
��������⣩
�������ʯ��ʯ����Ҫ�ɷ�ΪCaCO3����ϡ���ᷴӦ���ⶨ����CO2����������������������ݡ�
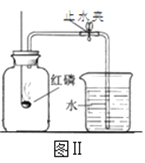
��ʵ����ƣ�ͨ����������ʵ��ֱ�ⶨCO2�������������
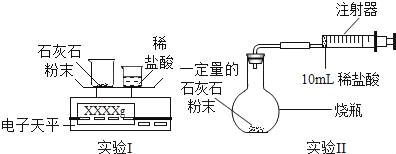
����������
��1����������ʵ���У���Ӧ�Ļ�ѧ����ʽ��_____��
��2��ʵ����У���С�ձ��е�����ϡ����ּ��μ��뵽���ձ��У������Ͻ��裬�ж�ʯ��ʯ��CaCO3��ȫ��Ӧ��ʵ��������_____��
��3��ʵ����У������Ӻ�װ�ã���_____����������ƣ���Ȼ��װ��ҩƷ�����10mlϡ�������������ƿ�С���ϡ�����ǻ�������ģ��������ɵĺ����
_____��
����¼�봦����
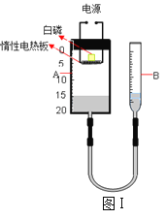
��4����֪ʵ��I��Ӧǰ��������[m�����ձ�+ʯ��ʯ��ĩ��+m��С�ձ�+ϡ���ᣩ]��Ҫ��������CO2�����������ٻ���Ҫ��������_____��
A m��С�ձ��� B m�����ձ��� C m�����ձ�+��Ӧ��ʣ���
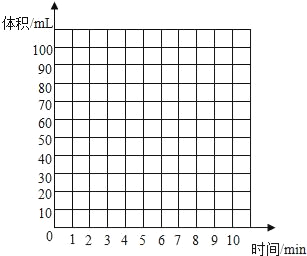
��5��ʵ����ʵ���¼���£�������������ͬ�¶ȡ���ͬѹǿ�����²ⶨ����
ʱ��/min | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
ע��������/ml | 60.0 | 85.0 | 88.0 | 89.0 | 89.5 | 89.8 | 89.9 | 90.0 | 90.0 | 90.0 |
����������ʵ����̺������ۺϷ�������������CO2�������_____mL��������_____��
�����������ʶ��������ͼ�л��Ƴ�0��10min����CO2�����ʱ��仯�����ߡ�
����˼�����ۣ�
��6����������������Ϊʵ��I/span>���ŵ���_____��ʵ�����ŵ���_____��