��Ŀ����
����Ŀ��ij��ѧС�������ұ����������ʴ�������������ʵ��̽����
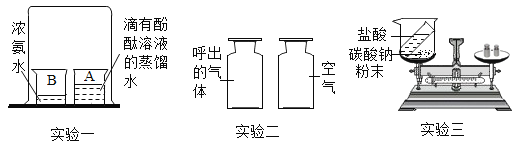
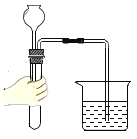
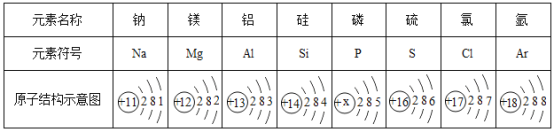
��1����С�������ͼ1�о�����ұ����
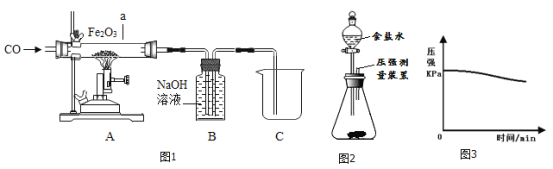
��ʵ���У�Ӧ_________(������A��������ͨCO��������ͨCO��A��������)��Ŀ����______��
��Ӳ�ʲ����������ֵ�����Ϊ______________��������Ӧ�Ļ�ѧ����ʽ��___��
��Bװ��_____���������������������������ռ�CO��
����a����������ȫ����ԭ��������ȴ���������������ȷ�Ӧǰ���������������2.4g�������a������������������___g��
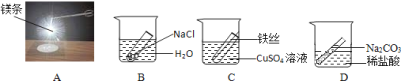
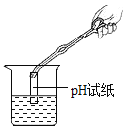
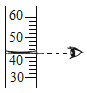
��2����С���������ͼ2װ�ã�����Ӧ��������������ƿ�ײ�������ƿ�����μ�����ʳ��ˮ����ʼ����������ѹǿ�ı仯��ѹǿ��ʱ��仯��ϵ��ͼ3��ʾ��
�����������������_____��_______�Ĺ�ͬ���á�ʵ����ʳ��ˮ��������____��
����ƿ��ѹǿ�½���ԭ����______��
���𰸡���ͨCO��A������ ����װ���ڿ�������ֹ����ʱ��ը ��ɫ������� 3CO+Fe2O3![]() 2Fe+3CO2 �� 8 ���� ˮ ����������ʴ װ��������������
2Fe+3CO2 �� 8 ���� ˮ ����������ʴ װ��������������
��������
��1��������һ����̼���п�ȼ�ԣ�ʵ���У�Ӧ��ͨCO��A�����ȣ�Ŀ���dz���װ���ڿ�������ֹ����ʱ��ը��
��������������һ����̼�ڸ��µ������·�Ӧ�������Ͷ�����̼������Ӳ�ʲ������г��ֵ�����Ϊ��ɫ������ڣ�������Ӧ�Ļ�ѧ����ʽ��3CO+Fe2O3![]() 2Fe+3CO2��
2Fe+3CO2��
�����ڶ�����̼�����������Ʒ�Ӧ��һ����̼�����������Ʒ�Ӧ������Bװ���������ռ�CO��
����a����������ȫ����ԭ��������ȴ���������������ȷ�Ӧǰ���������������2.4g�������a�����������������ǣ�2.4g��![]() ��100%=8g��
��100%=8g��
��2�������������������������ˮ�Ĺ�ͬ���ã�ʵ����ʳ��ˮ�������Ǽ���������ʴ��
����ƿ��ѹǿ�½���ԭ����װ�������������ģ�
�ʴ�Ϊ����1������ͨCO��A�����ȣ�Ŀ��������װ���ڿ�������ֹ����ʱ��ը���ں�ɫ������ڣ���3CO+Fe2O3![]() 2Fe+3CO2���� ��8g��
2Fe+3CO2���� ��8g��
��2����������ˮ������������ʴ����װ�������������ģ�

 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д�