��Ŀ����
����Ŀ������˵����ȷ���ǣ���
A. ��������ԭ10g����ͭ������Ƭ�̺���ȴ�Ƶ�ʣ���������Ϊ8.4g����μӷ�Ӧ������ͭ��������8g
B. ��5gij������ȫ�ܽ���95gˮ�У�������Һ�����ʵ���������һ����5%
C. ��3g̼��6g�������ܱ������е�ȼ����ַ�Ӧ�������е�����Ϊ������
D. ��һ�������������ƺ��������ƵĻ������������ˮ�У�������Һ�м��������̼������Һ����ַ�Ӧ�����ɵij���������ԭ������������ȣ���ԭ��������������Ƶ���������Ϊ26%
���𰸡�AD
��������
A����μӷ�Ӧ��CuO��������x��
CuO+H2![]() Cu+H2O �����������
Cu+H2O �����������
80 64 16
x 10g-8.4g![]()
x=8g��ѡ����ȷ��
B����5gij������ȫ�ܽ���95gˮ�У�������Һ�����ʵ�����������һ����5%����Ϊ�е���������ˮ����ˮ��Ӧ�����µ����ʣ�ʹ���ʵ���������5g���е�������ˮ��Ӧ���������ɳ��������壬�Ӷ�ʹ��Һ������С��100g������������Һ�����ʵ�����������һ����5%��ѡ�����
C����3g̼��ȫȼ�����ɶ�����̼ʱ������������Ϊx��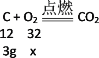
![]()
x=8g
��3g̼����ȫȼ������һ����̼ʱ������������Ϊy��
2C + O2 ![]() 2CO
2CO
2432
3gy![]()
y=4g
��Ϊ�ܱ���������6g��������8g��6g��4g����ȷ����Ӧ�����ɵļ���һ����̼Ҳ�ж�����̼��ѡ�����
D����������̼�������Ϊ100g�������������Ƶ�����Ϊx
Ca��OH��2+Na2CO3�TCaCO3��+2NaOH
74 100
x 100g![]()
x=74g
��������֪���������Ƶ�����Ϊ��100g-74=26g
�������Ƶ���������Ϊ��![]() ��100%=26%��ѡ����ȷ����ѡAD��
��100%=26%��ѡ����ȷ����ѡAD��

 ��������������������ϵ�д�
��������������������ϵ�д�