��Ŀ����
�����зḻ��ʯ��ʯ��Դ��ijͬѧΪ�˲ⶨij��ʯ��ʯ��̼��Ƶ�����������ȡ7.5g��Ʒ�����ձ��������������Ӧ��������������ϡ���������ٲ������壬�ų��������ڳ��������Ϊ1.1��
��1��������CO2������ܶ�Ϊ2.0g/������������Ӧ�ų����������Ϊ g��
��2����ʯ��ʯ�е����ʾ�������ˮ�Ҳ������ᷴӦ������ʯ��ʯ��CaCO3�������������������ս������1λС��������ȷ��0.1%����
��1��������CO2������ܶ�Ϊ2.0g/������������Ӧ�ų����������Ϊ
��2����ʯ��ʯ�е����ʾ�������ˮ�Ҳ������ᷴӦ������ʯ��ʯ��CaCO3�������������������ս������1λС��������ȷ��0.1%����
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���
ר�⣺�йػ�ѧ����ʽ�ļ���
���������ݶ�����̼��������ܶȿ��Լ��������̼�����������ݶ�����̼���������Լ���̼��Ƶ���������һ�����Լ���ʯ��ʯ��CaCO3������������
����⣺��1�����ɶ�����̼������Ϊ��2.0g/L��1.1L=2.2g��
���2.2��
��2����̼��Ƶ�����Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2����
100 44
x 2.2g
=
��
x=5g��
ʯ��ʯ��CaCO3����������Ϊ��
��100%=66.7%��
��ʯ��ʯ��CaCO3����������Ϊ66.7%��
���2.2��
��2����̼��Ƶ�����Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2����
100 44
x 2.2g
| 100 |
| x |
| 44 |
| 2.2g |
x=5g��
ʯ��ʯ��CaCO3����������Ϊ��
| 5g |
| 7.5g |
��ʯ��ʯ��CaCO3����������Ϊ66.7%��
������������Ҫ����ѧ�����ü��跨�ͻ�ѧ����ʽ���м�����ƶϵ�����������ʱҪע��淶�Ժ�ȷ�ԣ�

��ϰ��ϵ�д�
 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
�����Ŀ
����ͲȡҺ��ʱ���ӣ�����Ϊ15mL������ȡ��ʵ�������������
| A������15mL |
| B����15mL |
| C������15mL |
| D����ȷ�� |
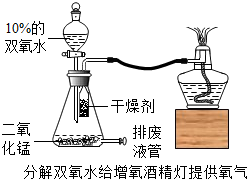 �����л�ѧ��ʦ����ʦ����������ͼ��ʾ�������ƾ��ƣ�ʹ��ʱ���������̴�˫��ˮ����������ͨ���о���������ף���ʱ�ܹ۲쵽���ĵľƾ�����ȼ�գ����淢�ף�
�����л�ѧ��ʦ����ʦ����������ͼ��ʾ�������ƾ��ƣ�ʹ��ʱ���������̴�˫��ˮ����������ͨ���о���������ף���ʱ�ܹ۲쵽���ĵľƾ�����ȼ�գ����淢�ף�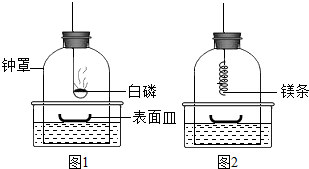 ʵ����ϣ�Ϊ����þ���ܷ���N2��ȼ�գ���ȤС���ͬѧչ������̽����
ʵ����ϣ�Ϊ����þ���ܷ���N2��ȼ�գ���ȤС���ͬѧչ������̽����