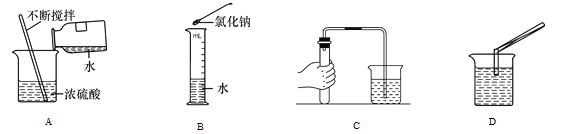
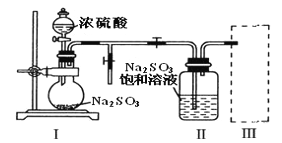
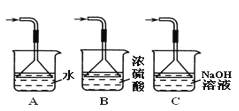
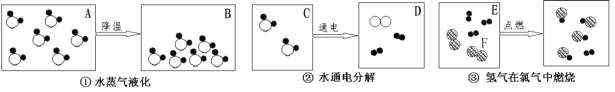
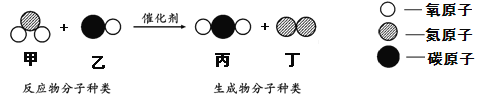
��Ŀ����
����Ŀ��ij��ѧ��ȤС���ͬѧΪ�˲ⶨʯ��ʯ��̼��Ƶ�������������ȡ��5��ʯ��ʯ��Ʒ�����ʲ���ϡ���ᷴӦ�����ֱ���������ϡ�������ʵ�飬������£�
ʵ�� | 1 | 2 | 3 | 4 | 5 |
ʯ��ʯ��Ʒ������/g | 1.25 | 2.50 | 5.00 | 6.25 | 7.50 |
����CO2������/g | 0.44 | 0.88 | 1.66 | 2.20 | 2.64 |
��������ʵ�����ݲ��ش�:
(1)��______��ʵ�����������Դ���������________________��
(2)��������ʯ��ʯ��Ʒ��̼��Ƶ���������______��
���𰸡��� ��Ϊ���������ʯ��ʯ�����������ɵĶ�����̼��������������� 80%
��������
��1���Ա����ʵ������Ʒ���������ɶ�����̼������������ϵ�����������Բ����Ϲ��ɵ�ʵ�飻
��2������̼�����ȫ��Ӧ��ʵ��������ȷ��ʵ���е����ݣ����ݷ�Ӧ�Ļ�ѧ����ʽ��������������������Ʒ��̼���������̼�����������Ʒ�����ȿɼ�������ʯ��ʯ��Ʒ��̼��Ƶ�����������
�⣺��1�����ݼ�¼���ݿɷ��֣���1��2��4��5��ʵ���У�����Ʒ�����ɱ�������������ᷴӦ�����������̼������Ҳ��Ӧ�ɱ����ӣ�˵�����������ʯ��ʯ�����������ɵĶ�����̼��������������ȣ�����3��ʵ���¼�Ķ�����̼������ȴ��������һ���ɣ���ˣ����жϴ˴μ�¼������������
��2����̼��Ƶ���������Ϊx��
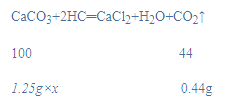
![]()
x=80%��
��ʯ��ʯ��Ʒ��̼��Ƶ�����������80%��

 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�