��Ŀ����
����Ŀ��2009�������������ڸ籾�������С��й��������ڼ��ŵij�ŵ�����չʾ���й�ı��չ���ٺ����������εĴ������Ŀǰ������̼������Ϊ����Ĺ�ʶ��
(1)CO2�������࣬����ЧӦ��ǿ��
����ʮ������������CO2�����������Ҫԭ���� ��
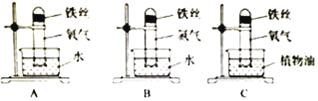
(2)���ٴ����ж�����̼�������о���
���������еĶ�����̼���͵������������ء�������̼����е��ܽ�ȱ�ͨ���������ˮ�е��ܽ�ȴ�ԭ���� �� ���п�ѧ�ҵ��������������Ӻ�ˮ����ȣ����º�������������������̼ʹ��ˮ��� ���ӵ�ԭ���� (�û�ѧ����ʽ��ʾ)��
��������Ķ�����̼�������ڴ����ͼ��ȵ������·�Ӧ��ת��Ϊˮ�ͼ��顣�����Ӧ�Ļ�ѧ����ʽΪ ��
(3)���ܼ��ţ���̼���
������3��28�հ�ɽ���������������������һСʱ���������Ϩ��һСʱ������˵������������ҪĿ�IJ�����ϵ��� ��
A����Լ��Դ �� B���������������ŷ� C�����ͳ���ҹ�� D����עȫ������
����̼�����ϸ���������һ����̼�ٴ�������������������������������������
���𰸡�(6��)(1)��ʯȼ�ϵ�ȼ�պ�ɭ���ҿ��ķ�
(2)��CO2���ܽ�����¶Ƚ��ͺ�ѹǿ��������� CO2+ H2O��H2CO3
![]()
(3)��C �����ֹص�(��˫����ֽ�����������𰸾���)
����������������1�������ж�����̼��Ҫ���ɹ���������ģ������IJ�������Ҫ����Ϊ�����Ŀ�ʯȼ��ȼ����ɵģ�
��2�������������ܽ�����¶ȵĹ�ϵ�����������̼����ˮ��Ӧ����̼�ᣬʹ��Һ�����ԣ���������Ϣ��������Ķ�����̼��������һ�������·�Ӧת���ɼ����ˮ����
��3���������ѧϰ�Ļ���֪ʶ����˼��������Ҫ����������IJ�����Ӱ������Ƕ���չ���������ճ������н��ܼ��ŵ�ʵ���ش�
����⣺��1���������ʹ��ú��ʯ�͵Ȼ�ʯȼ�ϣ�����ɴ����ж�����̼�������ӵ���Ҫԭ��
��2�������������ܽ�����¶ȵĹ�ϵ�������¶ȽϵͶ�����̼�ܽ�����ߣ��Ҷ�����̼����ˮ��Ӧ����̼�ᣬʹ��Һ�����ԣ�ʹ��ˮ������ǿ����������Ϣ��������Ķ�����̼��������һ�������·�Ӧת���ɼ����ˮ����д�йصķ���ʽ��
��3����������һСʱ���������������Ȼ����ᷢ���ÿ��3��28������Ϩ��һСʱ����������Լ���ܣ����ٻ�ʯȼ�ϵ�ʹ�ã���A��ȷ����A�з�����֪���ܽ�Լ�ˣ�ʹ�û�ʯȼ��Ҳ�����ˣ�����������ŷ���Ҫ�뻯ʯȼ�ϵĴ���ʹ���йأ�����B��ȷ�����ͳ���ҹ��������ҹ����Ҫ���ɸ��ֵƹ������ɵģ�����Ҫ���ĺܶ���ܣ���Ŀ�IJ��������C��������������Ҫ��ָ������̼�����壬���ǵĴ����ŷţ����µ�������¶����ߣ����������ڻ�����ƽ�����ߡ������ŵ���������һϵ���������⣬��D˵����ȷ���������ճ������н��ܼ��ŵ�ʵ���ش�
�ʴ�Ϊ����1����ʯȼ�ϵĴ���ʹ�û�ɭ�ֵ��ҿ��ķ�����2��CO2���ܽ�����¶Ƚ��ͺ�ѹǿ���������CO2+H2O=H2CO3��![]() ��3��C�����ֹصƣ����С��ﳵ����˳����л�ʹ���־�������ֽ��ʹ��һ����ľ����̻����Լ��ֽ��ʹ��ֽ�ʺؿ��ȣ�
��3��C�����ֹصƣ����С��ﳵ����˳����л�ʹ���־�������ֽ��ʹ��һ����ľ����̻����Լ��ֽ��ʹ��ֽ�ʺؿ��ȣ�

 �»����ܶ�Ա��ϵ�д�
�»����ܶ�Ա��ϵ�д� ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�