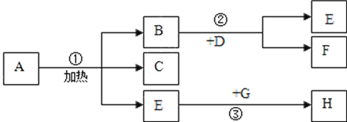��Ŀ����
����Ŀ���������ϵ��о���Ӧ���ǻ�ѧѧϰ��һ����Ҫ���ݣ�
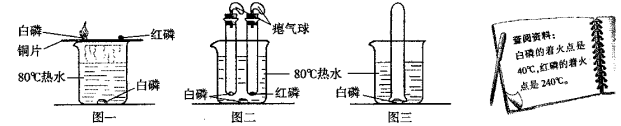
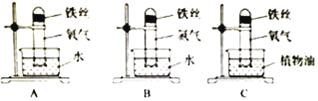
(1)Ϊ��̽������Ʒ��ʴ������������ͼ�����Ա�ʵ�飬һ�ܺ�B��C������������A�е�������_______________________��_____________________��ʵ��֤��������Ʒ��ʴ��Ҫ����������е�________________��ͬ���õĽ����
(2)����Ʒ��ʴ����ϡ�����ȥ���⣬������Ӧ�Ļ�ѧ����ʽΪ___________________________��
(3)���꣬����Ͷ�������������������������������г�ʹ���˴��������Ͻ𣬸úϽ�������Ⱦ��е��ŵ���________________________��
���𰸡� ��˿���� �Թ���Һ������ ������ˮ���� Fe2O3+3H2SO4�TFe2(SO4)3+3H2O Ӳ�ȴ���ʴ��
����������1�������������������������2�����ݻ�ѧ����ʽ����д������������3�����ݺϽ�����ʽ��з�������1��һ�ܺ�B��C������������A�е���������˿���⣬�Թ���Һ��������ʵ��֤��������Ʒ��ʴ��Ҫ����������е�������ˮ������ͬ���õĽ������2������Ʒ��ʴ����ϡ�����ȥ���⣬������Ӧ�Ļ�ѧ����ʽΪFe2O3+3H2SO4�TFe2��SO4��3+3H2O����3�����Ͻ�������Ⱦ��е��ŵ���Ӳ�ȴ���ʴ����