��Ŀ����
����Ŀ��ij��ѧ�о�С��Ե��ص�ʯ��ʯ��Ʒ�����о�����ȡʯ��ʯ��Ʒ8g����50gϡ�������μ�����Ʒ�У����ʵ�����ݼ��±�������ʯ��ʯ��Ʒ�е����ʲ�����ˮ���������ᷴӦ��������̼���ܽ⣬�Ȼ���������ˮ����
ʵ����� | ����ϡ���������/g | ʣ����������/g |
��1�� | 10 | 6 |
��2�� | 10 | 4 |
��3�� | 10 | 2 |
��4�� | 10 | 1 |
��5�� | 10 | m |
��ش𣺣�1����5��ʣ����������m=__________g��
��2��ʯ��ʯ��Ʒ��CaCO3����������Ϊ����_________����д��������̣���ͬ��
��3����Ӧ����������CO2�������Ƕ���_________��
���𰸡�1 87.5% 3.08g
��������
��1���ɱ���֪��ǰ����ʵ���У�ÿ����10gϡ�������Ĺ����������Ϊ2g�����Ĵμ���10gϡ����ֻ������1g���壬˵�����Ĵ�ʵ��ʱ�������ᷴӦ�Ĺ�����ȫ��Ӧ�ˣ�ͬʱ������ʣ�࣬������ʵ���е����������û����巴Ӧ����ʱʣ��Ĺ�����Ϊ1g������1��
��2���⣺ʯ��ʯ��Ʒ��CaCO3����������Ϊ![]() ��100��=87.5��
��100��=87.5��
��ʯ��ʯ��Ʒ��CaCO3����������Ϊ87.5����
��3���⣺������CO2������Ϊx
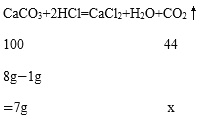
100:44=7g:x
x=3.08g
������CO2������Ϊ3.08g��

����Ŀ��Ϊ̽��̼��ԭ����ͭ�����ʵ����������ľ̿�ۺ�����ͭ�ĸ�������1~2.5g����ϵ��ʵ�顣
���������ϣ�������ͭ��CuO��Ϊ��ɫ���塣��̼��ԭ����ͭ�õ���ͭ�п��ܺ���������������ͭ��������ͭΪ��ɫ���壬
������ʵ�飩
ʵ��1��ȡ������1:11��ľ̿�ۺ�����ͭ�����1.3g������ʵ�顣
��� | 1��1 | 1��2 |
װ�� |
|
|
��Ӧ�����ʵ���ɫ��״̬ | ��ɫ��ĩ�л���������ɫ���� | ��ɫ�����н��������м�������ɫ���� |
ʵ��2��ȡһ�����Ļ�����1��2װ�ý���ʵ�顣
��� | ľ̿��������ͭ�������� | ��Ӧ�����ʵ���ɫ��״̬ | |
2��1 | 1:9 | ��ɫ�����н������� | ����������ɫ���� |
2��2 | 1:10 | ���к�������ɫ���� | |
2��3 | 1:11 | ���м�������ɫ���� | |
2��4 | 1:12 | ��ɫ���� | |
2��5 | 1:13 | ���н϶��ɫ���� | |
����������ۣ�
��1����ƽ��ѧ����ʽ����C+��CuO���� ��Cu+ ��CO2��______�������Ӧ��CuO��______����
��2��ʵ��1��2�У�֤���˲�����CO2��������__________��д����ѧ����ʽ______________
��3��ʵ��1��Ŀ����___________��
��4��ʵ��2�Ľ����ǣ�ľ̿������ͭ��Ӧ�����������Ϊ_______��
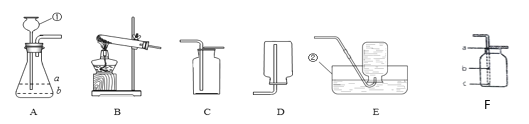
����Ŀ��������ͼ��ʾװ�ÿ��Խ���ʵ�����Ʊ����塣
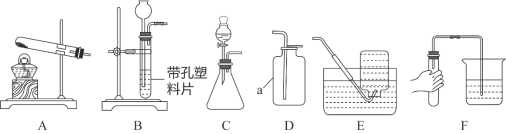
��1��ʵ������KMnO4�Ʊ�O2�Ļ�ѧ����ʽ��______________________________��
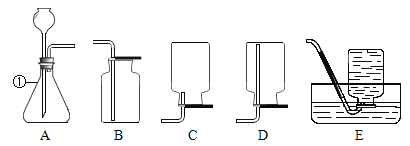
��2��ѡ�������ռ�O2��װ�ã���д���±��У�����ĸ����
ѡ��ҩƷ | ����װ�� | �ռ�װ�� |
H2O2��Һ��MnO2 | ______ | �ռ��ϴ���O2_______ |
KClO3��MnO2 | ______ | �ռ��ϸ���O2______ |
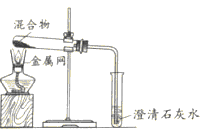
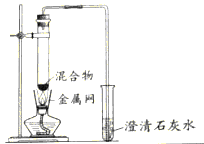
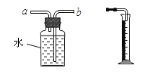
��3��ʵ����������װ��C��ȡCO2ʱ���÷�Ӧ���ɶ�����̼�г����������Ȼ������壬ijͬѧ��ͨ��ͼһװ��̽���Ƿ���HCl���壬�ڹ��ƿ��ʢ�ŵ��Լ�����������Һ������Ϊ�������Ӧ��__________������a������b�������롣
![]()
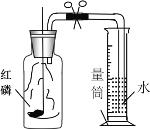
��4����ȡ����ǰӦ�ȼ��������ԣ�����Fͼ��ʾ����������ڵ��ܿ�δ�������ݣ������ԭ�������������ص���__________��ѡ���ţ���
A�����ձ�����ˮ�� B������������ˮ��λ�ù���������ݳ�
C���Թܿ���Ƥ��δ���� D����������ס�Թܣ��ٽ�������һ�˲���ˮ��