��Ŀ����
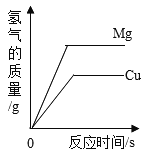
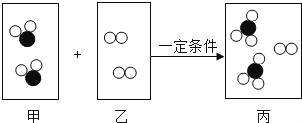
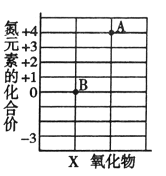
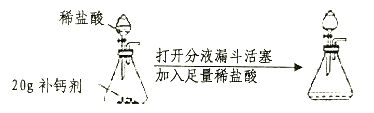
����Ŀ��С���С��ͬѧ��ѧϰ�ˡ������غ㶨�ɡ������ǵ�ʵ����������̽��������ѧ��Ӧ�Ƿ�Ҳ���������غ㶨�ɣ�С���С�Ƶ�ʵ�鷽����ͼ��ʾ��
������ʹ��ƽƽ�⣬�Ƴ��ձ���С�Թܡ�ҩƷ����������Ȼ��С�Թ����ҩƷ�����ձ��У��۲�������Ӧ��ȫ���ٴγ�����������������������һ��̽����
��1��С����ʵ������пɹ۲쵽�������Ǣ�_____________����_____________��С����ʵ������пɹ۲쵽�������Ǣ�_____________����_____________��
��2��������ʵ����������Ӧ�Ļ�ѧ����ʽΪ��
С��ͬѧ��ʵ�飺 _______________________________________��
С��ͬѧ��ʵ�飺_______________________________________��
��3��С���С�Ƹ��ݸ��Ե�ʵ������ó��˲�ͬ�Ľ��ۣ����ǵ�ʵ�����ƣ�Ϊʲô���۲�ͬ����ԭ����____________________________________________________��
��4��ʵ�������С���С��ͬѧ�������ܽᣬ�ó����ۣ��������������ɻ�������μӵķ�Ӧ��Ҫ����֤�����غ㶨�ɣ�������____________��ϵ�н��С�
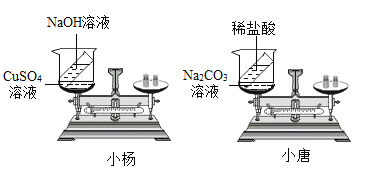
���𰸡�������ɫ���� ��ƽ����ƽ�� �����ݲ��� ��ƽָ������ƫת����ƽ�⣩ CuSO4+2NaOH==Na2SO4+Cu(OH)2�� Na2CO3+2HCl==2NaCl+H2O+CO2�� С���ʵ��û���������ɣ���С�Ƶ�ʵ���ڳ��������ڽ��еģ�С�Ƶ�ʵ��©����������������������������ɣ� �ܱ�.
��������
��1��С���ʵ��������μӻ����ɣ�CuSO4+2NaOH==Na2SO4+Cu(OH)2����Cu(OH)2����ɫ���������Ըù����пɹ۲쵽�������Dz�����ɫ��������ƽ����ƽ�⡣С�Ƶ�ʵ����������������ɣ����ɵ�������ɢ��������ȥ�ˣ�������ƽ��˵��������٣����Կɹ۲쵽�������������ݲ�������ƽָ������ƫת��
��2��С��ͬѧʵ��Ļ�ѧ����ʽΪ��CuSO4+2NaOH==Na2SO4+Cu(OH)2����С��ͬѧʵ��Ļ�ѧ����ʽΪ��Na2CO3+2HCl==2NaCl+H2O+CO2��
��3��С���ʵ��û���������ɣ���С�Ƶ�ʵ�����ڳ��������ڽ��еģ�С�Ƶ�ʵ��©������������������������������ǵ�ʵ�����ƣ�����ȴ��ͬ��
��4���������������ɻ�������μӵķ�Ӧ��Ҫ����֤�����غ㶨�ɣ��������ܱ���ϵ�н��С�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��С������![]() ��Һ��
��Һ��![]() ��ʵ��̽����������й��̣��ش��й����⡣
��ʵ��̽����������й��̣��ش��й����⡣
��1��![]() ��������5mL 5%��
��������5mL 5%��![]() ��Һ�м�������
��Һ�м�������![]() �����������������ݡ�
�����������������ݡ�
��д����![]() ��Һ�Ʊ�
��Һ�Ʊ�![]() �Ļ�ѧ����ʽ��______��
�Ļ�ѧ����ʽ��______��
����������Ӧԭ���Ʊ����ռ�һƿ�����![]() ��������װ��ͼ��ѡ����װһ��װ�ã�������˳��Ϊ______��C������
��������װ��ͼ��ѡ����װһ��װ�ã�������˳��Ϊ______��C������![]() ����______�����ţ���Ϊ��ȷ��ʵ��ɹ�����װҩƷ֮ǰӦ��______��
����______�����ţ���Ϊ��ȷ��ʵ��ɹ�����װҩƷ֮ǰӦ��______��
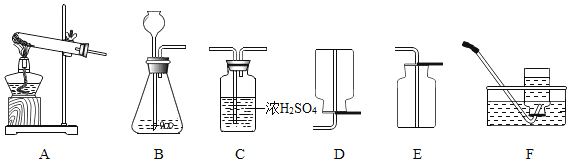
�ۼ���![]() �ķ�����______����ľ����ȼ����֤���ռ�������Ϊ
�ķ�����______����ľ����ȼ����֤���ռ�������Ϊ![]() ��
��
��2��![]() ��Һ��
��Һ��![]() �ֽ�Ĵ���
�ֽ�Ĵ���
��5mL5%��![]() ��Һ�м���2��һ��Ũ�ȵ�
��Һ�м���2��һ��Ũ�ȵ�![]() ��Һ�����������������ݡ�
��Һ�����������������ݡ�
����֪��![]() ��Һ����Ҫ������������
��Һ����Ҫ������������![]() ��
��![]() ��
��![]()
�����⣩��������![]() ��Һ�ķֽ�������ã�
��Һ�ķֽ�������ã�
�����裩����һ��������![]() ��
��
�������������![]()
��������������![]()
���������ټ���һ�����ܳ�����������______��
��ʵ�飩
���� | ���� |
�����������䣬�� | �����Ա仯 |
�����������䣬�� | �����Ա仯 |
�����������䣬�� | ���������������� |
�����ۣ��ڼ���______������������һ����һ�ּ��費������
��3�������Ƚ�
��ѭ�����õĽǶȷ�����______���ѧʽ�����ʺ����÷�Ӧ�Ĵ�����
����Ŀ�����ʱ�����Ҫ�Ļ�����Ʒ���ڹ�����ռ����Ҫ��λ����ҵ���ü��Һϳ��ڡ��ס��ҡ��ڵ���ʾ��ͼ���±���
���� | �� | �� | �� |
|
��ʾ��ͼ |
|
|
|
��l���ס��ҡ��������ڻ�������ǣ�����ţ�______��
��2����֪���ʱ�����Ԫ�صĻ��ϼ�Ϊ![]() ������һ��Ԫ�صĻ��ϼ�Ϊ______��
������һ��Ԫ�صĻ��ϼ�Ϊ______��
��3������![]() �ļ�
�ļ�![]() ���һ�Ϸ�Ӧ��ȡ�ڣ���Ӧһ��ʱ����ⶨʣ�������Ϊ
���һ�Ϸ�Ӧ��ȡ�ڣ���Ӧһ��ʱ����ⶨʣ�������Ϊ![]() ����ʣ���ҵ�����Ϊ______g��
����ʣ���ҵ�����Ϊ______g��
����Ŀ����ѧ��ȤС����ʵ����������غͶ������̻�ϼ�����ȡ������ij��ʵ��ʱżȻ�����Ƶõ������д̼�����ζ����һ����������ͬѧ�ǵ���Ȥ����������ʦ��ָ���¶Ը�����ɷֽ���̽����
��������⣩�������������̻�ϼ��Ⱥ����������ɷ���ʲô��
���������ϣ�
���������������̻�ϼ��Ȳ���������ֻ�Ե�����ʽ���ڣ�
��������![]() �����д̼�����ζ�����壬������ˮ����ʹʪ��ĵ��۵⻯����ֽ������
�����д̼�����ζ�����壬������ˮ����ʹʪ��ĵ��۵⻯����ֽ������
��������裩
����һ��������Ϊ![]() ���������������Ϊ______����������������Ϊ
���������������Ϊ______����������������Ϊ![]() ��
��![]() �Ļ���
�Ļ���
��ʵ��̽����
��� | ���� | ���� | ���� |
����I | ����ˮ�������ռ��ס�����ƿ���壬�������ǵ�ľ�������ƿ��ʪ��ĵ��۵⻯����ֽ������ƿ�� | ľ����ȼ�� ���۵⻯����ֽ����ɫ | ����______���� |
����II | �����ſ������ռ��ס�����ƿ���壬�������ǵ�ľ�������ƿ��ʪ��ĵ��۵⻯����ֽ������ƿ�� | ľ��______�� ���۵⻯����ֽΪ______ɫ | ���������� |
����˼���ۣ�
Ϊʲô���ַ����ó��Ľ��۲�һ�£��ĸ�����ȷ�ģ�
ͬѧ������I���۲���ȷ���������������������֮����______��
��ʦ������Ҫ����ʵ�飬�����ų�����һ��������______��
����չӦ�ã�![]() ����������������������Ӧ�ʵ����ͨ����
����������������������Ӧ�ʵ����ͨ����![]() ��Ũ���Ṳ����ȡ
��Ũ���Ṳ����ȡ![]() ��ͬʱ����
��ͬʱ����![]() �۵��̵��Ȼ������һ�ֳ����Ļ������д���÷�Ӧ�Ļ�ѧ����ʽ��______��
�۵��̵��Ȼ������һ�ֳ����Ļ������д���÷�Ӧ�Ļ�ѧ����ʽ��______��