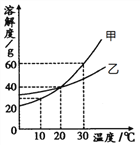
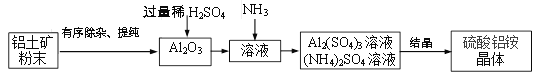
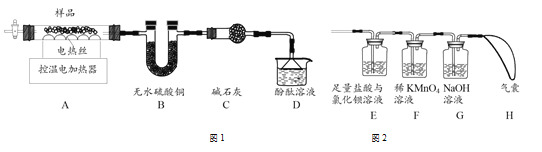
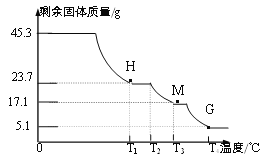
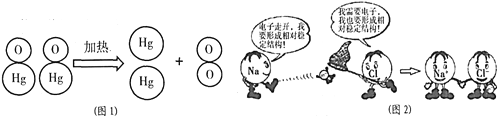
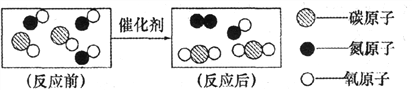
��Ŀ����
����Ŀ���ҹ�����������֪��������Ƚ���ȫ����������֮һ�����ﴢ��Ҳ�ܷḻ�����������һ�ֳ����Ľ�����ʯ��Ϊ�ⶨһ����������Ʒ����Ҫ�ɷ���Al2O3����������ɷֲ����뷴Ӧ��Ҳ������ˮ���Ĵ��ȣ�ͬѧ��������ͼ��ʾ��ʵ�飺
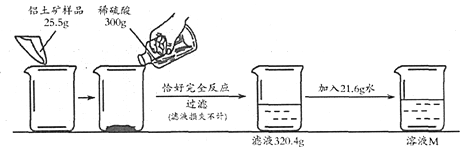
��ش�
��1��ʵ���з�����Ӧ�Ļ�ѧ����ʽΪ_____________________��
��2�����ݼ�֪�������г����ʵ���вμӷ�Ӧ�������������x���ı���ʽΪ_______��
��3��������������Ʒ�Ĵ��ȣ�Al2O3������������Ϊ________��
��4��ʵ�����õ�ϡ��������ʵ���������Ϊ________��
��5��ʵ���������õ�����ҺM�У����ʺ��ܼ����������������Ϊ_______��
��6������ҵ��������135t����80�������Ͻ���Ҫ���������������������_______����������������⣬����ɷ־�������Ԫ�أ����ƹ�����û����Ԫ����ʧ��
���𰸡� Al2O3 + 3H2SO4![]() Al2(SO4)3 + 3H2O
Al2(SO4)3 + 3H2O ![]() 80% 19.6% 1:4 255t
80% 19.6% 1:4 255t
����������1����������ϡ���ᷴӦ������������ˮ����ѧ����ʽΪ��Al2O3+3H2SO4�TAl2��SO4��3+3H2O��
��2���⣺��μӷ�Ӧ�����������Ϊx������������������Ϊy��
�������غ㶨�ɿ�֪���μӷ�Ӧ��������������Ϊ320.4g-300g=20.4g
Al2O3+3H2SO4�TAl2��SO4��3+3H2O
102 294 342
20.4g x y
![]()
x=58.8g
![]()
y=68.4g
��3����������Ʒ�Ĵ���Ϊ![]() ��100%=80%��
��100%=80%��
��4��ʵ�����õ�ϡ��������ʵ���������Ϊ![]() ��100%=19.6%
��100%=19.6%
��5��ʵ���������õ�����ҺM�У����ʺ��ܼ����������������Ϊ68.4g����320.4g-68.4g+21.6g��=1:4��
��6������Ҫ���������������������z
Al2O3----------2Al
102 54
z��80% 135 t��80%
![]()
z=255t
�𣺣�3��������������Ʒ�Ĵ��ȣ�Al2O3������������Ϊ80%��
��4��ʵ�����õ�ϡ��������ʵ���������Ϊ19.6%��
��5��ʵ���������õ�����ҺM�У����ʺ��ܼ����������������Ϊ1:4��
��6������ҵ��������135t����80�������Ͻ���Ҫ���������������������255t��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ����֪M��N�ֱ���ϡ���ᡢ����������Һ�е�һ�֡�ij��ѧ��ȤС��ͬѧ��һ������M�в��ϵμ�N�����ⶨ������Һ��pH������ͼ��ʾ��
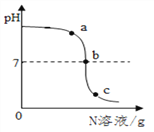
(1)M��______________��
(2)��Ӧ�����У���ʦ�ֱ�ȡͼ��a��b��c��������Ӧ����Һ������˳�����ͬѧ����һ���ķ�����������Һ���Լ���(���òⶨ��ҺpH�ķ���)��
С����С��ֱ�ȡ����һ����Һ����ʵ�飺С������ȡ��Һ�м���___________��Һ���۲쵽����ɫ�������ɡ�
���ۣ���a����Һ��
С����������һ����Һ�еμӷ�̪��Һ���۲쵽____________��
���ۣ���b����c����Һ��
Ϊ��һ��ȷ������Һ�ɷ֣�С������Ʋ��������ʵ�飺
ʵ�鲽�� | ʵ������ | ���ۼ���ѧ����ʽ |
ȡ������������������������Һ | ������ȫ�ܽ⣬��_____________ | ��صĻ�ѧ����ʽΪ_____________�� Ϊc����Һ������֪c����Һ�к��е�������_____________(��д���ӷ���) |
���ۣ�ʣ��һ��Ϊb����Һ��