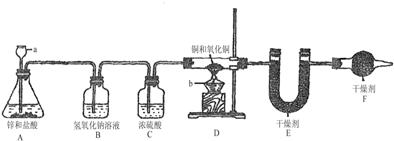
��Ŀ����
ij������Ʒ�к��������Ȼ��ƣ������ⶨ����̼���Ƶ�������������������ʵ�飺Na2CO3+H2SO4��Na2SO4+H2O+CO2����ͨ��ʵ���÷�Ӧ�����Ķ�����̼���������������ԭ��Ʒ��̼���Ƶ��������������̼��������Ʒ�е�����������
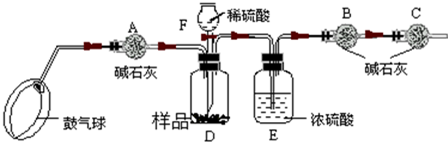
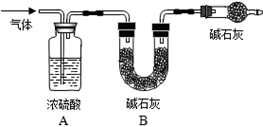
����ͼ����װ�ã���B��C�⣩����������ҩƷ��
�ڳ�������¼B��������m1����������ʱע����B�����ˣ�
�۰�����������Լ1���ӣ�
��������B��C��
�ݴ�Һ©��F�Ļ�������ϡ������ټ���D�кرջ�����
�ް�����������Լ1���ӣ�
�߳�������¼B��������m2����������ʱע����B�����˼�E�Ҷ˵ij��ڣ�
����㣺
��1����֪��ʯ�ҵ���Ҫ�ɷ����������ƺ��������ƣ�������A��������______�������C��������______��������й�����Ŀ����______��������й�����Ŀ����______����ʵ���ܷ�ͬʱʡ�Ԣۡ����������裿______��ԭ����______��
��2������ȡ��Ʒ������Ϊ5g��Ϊȷ��ʵ��˳�����У���Һ©��F������Ҫʢ��10%��ϡ���ᣨ��=1.07g/mL��______mL����m1Ϊ51.20g��m2Ϊ53.18g����Ʒ��̼���Ƶ���������Ϊ______��

����ͼ����װ�ã���B��C�⣩����������ҩƷ��
�ڳ�������¼B��������m1����������ʱע����B�����ˣ�
�۰�����������Լ1���ӣ�
��������B��C��
�ݴ�Һ©��F�Ļ�������ϡ������ټ���D�кرջ�����
�ް�����������Լ1���ӣ�
�߳�������¼B��������m2����������ʱע����B�����˼�E�Ҷ˵ij��ڣ�
����㣺
��1����֪��ʯ�ҵ���Ҫ�ɷ����������ƺ��������ƣ�������A��������______�������C��������______��������й�����Ŀ����______��������й�����Ŀ����______����ʵ���ܷ�ͬʱʡ�Ԣۡ����������裿______��ԭ����______��
��2������ȡ��Ʒ������Ϊ5g��Ϊȷ��ʵ��˳�����У���Һ©��F������Ҫʢ��10%��ϡ���ᣨ��=1.07g/mL��______mL����m1Ϊ51.20g��m2Ϊ53.18g����Ʒ��̼���Ƶ���������Ϊ______��
��1�������A�������չ������й���Ŀ����еĶ�����̼������Ը����B������������ɸ��ţ������C�ڸ����B֮������ֹ�����еĶ�����̼��ˮ�������ܣ�������й�����Ŀ�����ó�ȥ������̼�Ŀ���������ϵ�еĶ�����̼������������������ѹ����ʹ�����Ķ�����̼ȫ�����ų���Ҫ�ⶨ������̼�����������ų������ж�����̼�����ɵĶ�����̼��������Ӱ�죻��ʵ�鲻��ͬʱʡ�Ԣۡ����������裬������ʵ�������
��2����5����Ʒȫ��Ϊ̼����ʱ����������࣬���������������Ϊx��
Na2CO3+H2SO4�TNa2SO4+H2O+CO2��
10698
5g x
��
| 106 |
| 5g |
| 98 |
| x |
������Ҫʢ��10%��ϡ���ᣨ�ܶ�Ϊ1.07g/mL�������Ϊ��V=
| ||
| 1.07 |
�����������ݣ����ɶ�����̼������Ϊ��m2-m1��=1.98g����̼���Ƶ�����Ϊy�����У�
Na2CO3+H2SO4�TNa2SO4+CO2��+H2O
106 44
y 1.98
| 106 |
| y |
| 44 |
| 1.98g |
���Դ�����Ʒ����Ϊ��
| 4.77g |
| 5g |
�ʴ�Ϊ��
��1����ȥ��������еĶ�����̼����ֹ�����еĶ�����̼��ˮ����B��Ӱ��ʵ�������ó�ȥ������̼�Ŀ���������ϵ�еĶ�����̼���ó�ȥ������̼�Ŀ�������Ӧ�����Ķ�����̼ȫ������B�У����ܣ��ٿ����к�����������̼���ڷ�Ӧ��װ���в���������̼������ɽ���ƫ�
��2��43.0��95.4%��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ