��Ŀ����
��10�֣���ȡMnO2��KClO3�Ĺ�������15.25g�����������ٲ�������Ϊֹ�������Ⱥ�ʣ������ȴ�����£���40gˮ��4�μ���ʣ������г���ܽ⡣��ˮ��������ʣ�������������±���(MnO2�Dz�����ˮ�Ĺ����ĩ)
����1������mֵΪ ��
��2�����Ƶ�������������
��3��ʣ��������40gˮ���γ���Һ���ʵ�������������������ȷ��0.1%����
| ��� | l | 2 | 3 | 4 |
| ��ˮ������(g) | 10 | 10 | 10 | 10 |
| ʣ����������(g) | 7.25 | 4.05 | 3 | m |
��2�����Ƶ�������������
��3��ʣ��������40gˮ���γ���Һ���ʵ�������������������ȷ��0.1%����
��1��3�� ��2��4.8g�� ��3��15.7%
��ͼ����֪������10gˮ����ܽ�KCl�������͵��������Ĵμ�ˮ���ܽ�KCl���������ȽϿ�֪ʣ��Ĺ���ȫ���Dz�����ˮ��MnO2���ݴ˴��⣻
���ݻ�ѧ����ʽ�����Եó�������֮��������ȣ�д������ʽ���Ϳɼ���ó����Ƶ��������������Ȼ��ص�����
��2����3��KClO3������="15.25g-3g=12.25g" ��1�֣�
�跴Ӧ��������������Ϊx���������Ȼ�������Ϊy
2KClO3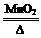 2KCl+3O2�� (1��)
2KCl+3O2�� (1��)
245 149 96
12.25g y x
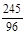 =
=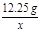 x=4.8g ��2�֣�
x=4.8g ��2�֣�
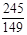 =
=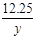 y=7.45g ��2�֣�
y=7.45g ��2�֣�
��2�����õ���������Ϊ4.8g
��3���γ��Ȼ�����Һ����������=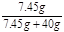 ��100%=15.7% ��2�֣�
��100%=15.7% ��2�֣�
���ݻ�ѧ����ʽ�����Եó�������֮��������ȣ�д������ʽ���Ϳɼ���ó����Ƶ��������������Ȼ��ص�����
��2����3��KClO3������="15.25g-3g=12.25g" ��1�֣�
�跴Ӧ��������������Ϊx���������Ȼ�������Ϊy
2KClO3
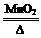 2KCl+3O2�� (1��)
2KCl+3O2�� (1��)245 149 96
12.25g y x
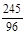 =
=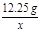 x=4.8g ��2�֣�
x=4.8g ��2�֣�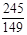 =
=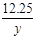 y=7.45g ��2�֣�
y=7.45g ��2�֣���2�����õ���������Ϊ4.8g
��3���γ��Ȼ�����Һ����������=
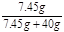 ��100%=15.7% ��2�֣�
��100%=15.7% ��2�֣�
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
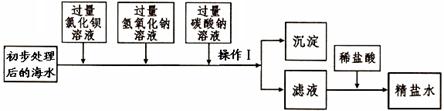
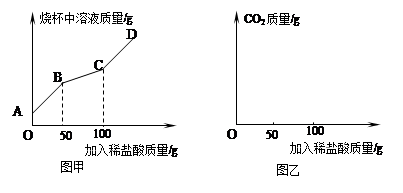

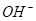 �������йأ�һ���������Һ��
�������йأ�һ���������Һ��